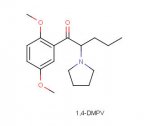
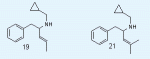Care to share what the structures of 19 and 21 are?The simple aletamine seems mainly a analgetic.
Clinical Evaluation of Aletamine as an Analgesic
Leo J. Cass M.D.1 and Willem S. Frederik M.D., Ph.D.2
1 University Health Services, Harvard University, Cambridge, Mass., and Visiting Physician, Long Island Hospital, Boston, Mass.
2 University Health Services, Harvard University, Cambridge, Mass.
The analgesic potency of aletamine hydrochloride was investigated in a doubleblind cross-over study with 35 patients. Aletamine hydrochloride was utilized at two dosage levels (250 and 125 mg. three times a day) and was compared with propoxyphene hydrochloride (65 mg. three times a day) and a placebo. Patients were evaluated three times a day, one hour after administration for relief of pain and once each day for changes in mood. Side effects observed or spontaneously expressed by the patients were also recorded.
The results of this study indicate that, given three times daily, aletamine hydrochloride, 250 mg., has a greater analgesic potency than propoxyphene hydrochloride, 65 mg., or aletamine hydrochloride, 125 mg. Aletamine hydrochloride, 125 mg., produces an analgesic effect almost equivalent to that of propoxyphene hydrochloride, 65 mg. Only minor changes in mood were evident for any of the treatments. The incidence of side effects was relatively low for each of the four drug regimens
http://jcp.sagepub.com/cgi/content/abstract/6/2/96