-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Phenmetrazine
- Thread starter Tryptamite
- Start date
I believe the above compound easier to make than mormamide (I don't see why a clankemist would seperate Dextro and levo instead of just doubling the dose of racemic! Unless levo has undesirable qualities, but as far as I know it is totally inactive)
Easier to make yes, but it's controlled by the UNODC so it's illegal EVERYWHERE. A couple of the less potent homologues were sold as RCs but their potency was LOW.
If one could obtain the direct precursor to dextromoramide, instead of adding a cyclopentylamine moiety, a pyrrolidine would work just as well. The bioavailability of dextromoramide orally is almost 100% so that or snorting (and that would need the sulfate of phosphate salt - they are much more water soluble) and the vendors are so poor these days, I doubt they would even manage THAT.
I'm not sure if it's the intermediate or the final compound that is resolved but racemoramide is nowhere near as good because the body is then absorbing something of which 50% is inactive. I mean, it's quite possible to resolve said intermediate, but vendors are LASY.
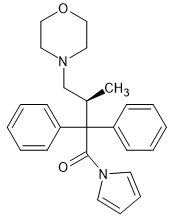
The fact that the cyclopentylamine is so much more active that closely related homologues and the fact that the methyl side chain is at the alpha not the beta suggests that a semi-rigid moiety is required. A ketone or ester moiety have rotatable bonds. What the amide does is to rotate one of the benzene rings slightly so it fits into the receptor PERFECTLY. A cis 22,33 tetra methyl azetidine may work or possible an (S,S) 2,4-dimethylaziridine. Their activity would allow the angle of that aromatic to be measured so that even more potent analogues can be found, but it was discovered in the 50s or the 60s and the fact that it's SO specific is still an amazing detail.
Like R-4066, it shows that high affinity is only part of the story. Receptor occupancy T½ is also key. I mean, without that ester, the R-4066 intermediate only has a duration of 3 hours (although it's potency is not noted). MAYBE in those 3 hours, it's potency is several thousand times that of M and the given figure is merely due to the fraction which is de-esterified? I do not know.
I remember a friend making a huge batch of 3,4-MD phenmetrazine...... and taking it upto 1000mg. It didn't work.
But recent studied of methylphenidate (Ritalin) show that p-Me phenmetrazine is very similar to mephedrone. Likewise, 2,5-dimethoxy-4-bromephenmetrazine has proved to be as active as it's PEA counterpart and so the question is - will 3,4MD phenmetrazine be MDMA-like.
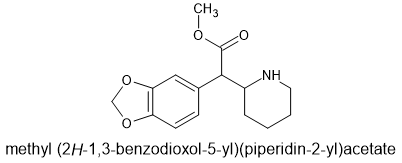
Now, When David Nichols et al produced the 2,5-dimethoxy-4-Br homologue, the ester function wasn't needed and so:
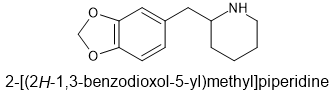
Maybe the above is sufficient. Reports on the unsubstituted homologue suggested that it had little DRI but will the MD solve that issue. If not, I haven't seen:
https://ibb.co/xDWsBgL
Anyone have any other ideas? Sorry to blab on......... but this is how my brain works.
But recent studied of methylphenidate (Ritalin) show that p-Me phenmetrazine is very similar to mephedrone. Likewise, 2,5-dimethoxy-4-bromephenmetrazine has proved to be as active as it's PEA counterpart and so the question is - will 3,4MD phenmetrazine be MDMA-like.

Now, When David Nichols et al produced the 2,5-dimethoxy-4-Br homologue, the ester function wasn't needed and so:

Maybe the above is sufficient. Reports on the unsubstituted homologue suggested that it had little DRI but will the MD solve that issue. If not, I haven't seen:
https://ibb.co/xDWsBgL
Anyone have any other ideas? Sorry to blab on......... but this is how my brain works.
paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
Wonder why the piperazines analogs havent been tried (recreationally or functional): I mean replacing the morpholine ring of phenmetrazine with a piperazine. According to the french patent on those (2-methyl-3-arylpiperazines), they are actually more potent than the morpholine homologs: in rats at 1mg/kg "substantial mydriasis... excitement...piloerection..etc for 3 hours".. looks like a very potent stim to me. Similar things in mice.
I mean 1mg/kg in rats would translate roughly to c.a. a 10mg dose for a 70kg human.
Now on the other hand, they did clinical trial in humans and suggest a dose of 100-150mg oral/day as "good antidepressant of melancholy type..etc". Wonder why the compound was not developed further: probably the subjects were tweaking pretty hard on that dose!??
Actually I wont be surprised the piperazines are more potent than the morpholines as DA-NE releasers because of the extra H-bond donor. If Phenmetrazines SAR is similar, the (+/-) trans but not the cis would be active. And the 2S,3S trans enantiomer more potent than the 2R,3R trans since much of the activity of phenmetrazine (as DA-NE releaser) is due to the 2S,3S isomer. Similar SAR with the 4-MAR oxazolidines (the trans isomer is more potent than the cis).
1-step synthesis from dirt cheap SMs looks even easier than the morpholines. Now If one can its hands on the trans isomer (+/-)trans-2-methyl-3-phenylpiperazine, I bet you it would be quite interesting compound, probably not much different from the holy grail trans-4-MAR but with reduced duration and reduced serotonergic activity.
nb: the safety of that compound is quite good at least in mice and rats (LD0 in mice>250mg/kg!! 250x active dose) but anyhow they did clinical trials in humans and suggest 150mg (oral) dose so I suspect it is well tolerated, but who knows?
Edit: if anybody come across some papers on those, please post .. Stay Safe All BLighters
I mean 1mg/kg in rats would translate roughly to c.a. a 10mg dose for a 70kg human.
Now on the other hand, they did clinical trial in humans and suggest a dose of 100-150mg oral/day as "good antidepressant of melancholy type..etc". Wonder why the compound was not developed further: probably the subjects were tweaking pretty hard on that dose!??
Actually I wont be surprised the piperazines are more potent than the morpholines as DA-NE releasers because of the extra H-bond donor. If Phenmetrazines SAR is similar, the (+/-) trans but not the cis would be active. And the 2S,3S trans enantiomer more potent than the 2R,3R trans since much of the activity of phenmetrazine (as DA-NE releaser) is due to the 2S,3S isomer. Similar SAR with the 4-MAR oxazolidines (the trans isomer is more potent than the cis).
1-step synthesis from dirt cheap SMs looks even easier than the morpholines. Now If one can its hands on the trans isomer (+/-)trans-2-methyl-3-phenylpiperazine, I bet you it would be quite interesting compound, probably not much different from the holy grail trans-4-MAR but with reduced duration and reduced serotonergic activity.
nb: the safety of that compound is quite good at least in mice and rats (LD0 in mice>250mg/kg!! 250x active dose) but anyhow they did clinical trials in humans and suggest 150mg (oral) dose so I suspect it is well tolerated, but who knows?
Edit: if anybody come across some papers on those, please post .. Stay Safe All BLighters
izo
Bluelighter
I think the m-F increases the SERT activity of the drug. The
Meta-fluoro has a certain serotonergic feel to it but not that drastic as with 3f-Amphetamine. 4methyl also a Little bit of serotonin action but also not that pronounced.
izo
Bluelighter
I'm told that the thiambutene opioids (a British invention but only used in Japan) are very similar to Palfium
They are reported to be very lackluster and are devoid of any real opioid euphoria.
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
Wonder why the piperazines analogs havent been tried (recreationally or functional): I mean replacing the morpholine ring of phenmetrazine with a piperazine. According to the french patent on those (2-methyl-3-arylpiperazines), they are actually more potent than the morpholine homologs: in rats at 1mg/kg "substantial mydriasis... excitement...piloerection..etc for 3 hours".. looks like a very potent stim to me. Similar things in mice.
I mean 1mg/kg in rats would translate roughly to c.a. a 10mg dose for a 70kg human.
Now on the other hand, they did clinical trial in humans and suggest a dose of 100-150mg oral/day as "good antidepressant of melancholy type..etc". Wonder why the compound was not developed further: probably the subjects were tweaking pretty hard on that dose!??
Actually I wont be surprised the piperazines are more potent than the morpholines as DA-NE releasers because of the extra H-bond donor. If Phenmetrazines SAR is similar, the (+/-) trans but not the cis would be active. And the 2S,3S trans enantiomer more potent than the 2R,3R trans since much of the activity of phenmetrazine (as DA-NE releaser) is due to the 2S,3S isomer. Similar SAR with the 4-MAR oxazolidines (the trans isomer is more potent than the cis).
1-step synthesis from dirt cheap SMs looks even easier than the morpholines. Now If one can its hands on the trans isomer (+/-)trans-2-methyl-3-phenylpiperazine, I bet you it would be quite interesting compound, probably not much different from the holy grail trans-4-MAR but with reduced duration and reduced serotonergic activity.
nb: the safety of that compound is quite good at least in mice and rats (LD0 in mice>250mg/kg!! 250x active dose) but anyhow they did clinical trials in humans and suggest 150mg (oral) dose so I suspect it is well tolerated, but who knows?
Edit: if anybody come across some papers on those, please post .. Stay Safe All BLighters
A reference would be good. The benzhydramine scaffold is possibly the simplest in chemistry so LOTS of such compounds are active.
paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
US 4,912,110 (notice this route is not stereoselective. most likely gives mix of 4 diastereomers! ratio? I don't know)
doi: 10.1016/S0014-2999(02)01830-7
benzhydryl compounds also most likely to be toxic ie more off-targets interactions.. the most serious beeing cardiac HERG blockade!! pretty much a general rule (anything that contains benzhydryl and a basic amine will (most likely) block it!?)
doi: 10.1016/S0014-2999(02)01830-7
benzhydryl compounds also most likely to be toxic ie more off-targets interactions.. the most serious beeing cardiac HERG blockade!! pretty much a general rule (anything that contains benzhydryl and a basic amine will (most likely) block it!?)
fastandbulbous
Bluelight Crew
Once got a phone call, from a mate, saying one of his mates had IV'd a 10mg palfium. After quickly realizing he wasn't going to ring an ambulance, I suggested making an IV shot of amphetamine (they had just bought a ripped off pharmacy CD cabinet), put them in the recovery position, but with legs above head height (massive drop in B.P.). Twenty minutes later, I started a rant about how he nearly had a dead body on his hands and that dextromoramine should NEVER be IV'd...Fuckin hell. Peach palfium. Now there's a blast from the past.
Never had the pleasure myself, but the pharmaceuticals available in the 60s and 70s were insane.
Strange how we've managed to survive the good drugs, but it's the shit ones which are killing us.
@#GIMMESOMEPROPERDRUGSBECAUSEWHATIMGETTINGISFUCKINSHIT
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
Fencamfamine ha opioid activity. Some of it's effects are reversed by naloxone. I think camfentamine has less opioid activity.
If you overlay fencamfamine and (1S,2R) nortilidine, the benzene ring and N: overlay perfectly. If their was a way to incorporate a -CN or -OH to the benzylic carbon, it would only be 1 or 2 steps to an acetyl ester or a propionoxy moiety to that quaternary carbon.
If you overlay fencamfamine and (1S,2R) nortilidine, the benzene ring and N: overlay perfectly. If their was a way to incorporate a -CN or -OH to the benzylic carbon, it would only be 1 or 2 steps to an acetyl ester or a propionoxy moiety to that quaternary carbon.
fastandbulbous
Bluelight Crew
The morpholine compounds are more active than the piperidine rings, when it comes to methylphenidate derivatives (the morpholine analogue of desoxypipradrol is stupidly potent). To alter the half life of a stim drug, based on methylphenidate, replace the ester with a methoxy or even plain alkyl group, as that's the way the drug is deactivated metabolically.Wonder why the piperazines analogs havent been tried (recreationally or functional): I mean replacing the morpholine ring of phenmetrazine with a piperazine. According to the french patent on those (2-methyl-3-arylpiperazines), they are actually more potent than the morpholine homologs: in rats at 1mg/kg "substantial mydriasis... excitement...piloerection..etc for 3 hours".. looks like a very potent stim to me. Similar things in mice.
I mean 1mg/kg in rats would translate roughly to c.a. a 10mg dose for a 70kg human.
Now on the other hand, they did clinical trial in humans and suggest a dose of 100-150mg oral/day as "good antidepressant of melancholy type..etc". Wonder why the compound was not developed further: probably the subjects were tweaking pretty hard on that dose!??
Actually I wont be surprised the piperazines are more potent than the morpholines as DA-NE releasers because of the extra H-bond donor. If Phenmetrazines SAR is similar, the (+/-) trans but not the cis would be active. And the 2S,3S trans enantiomer more potent than the 2R,3R trans since much of the activity of phenmetrazine (as DA-NE releaser) is due to the 2S,3S isomer. Similar SAR with the 4-MAR oxazolidines (the trans isomer is more potent than the cis).
1-step synthesis from dirt cheap SMs looks even easier than the morpholines. Now If one can its hands on the trans isomer (+/-)trans-2-methyl-3-phenylpiperazine, I bet you it would be quite interesting compound, probably not much different from the holy grail trans-4-MAR but with reduced duration and reduced serotonergic activity.
nb: the safety of that compound is quite good at least in mice and rats (LD0 in mice>250mg/kg!! 250x active dose) but anyhow they did clinical trials in humans and suggest 150mg (oral) dose so I suspect it is well tolerated, but who knows?
Edit: if anybody come across some papers on those, please post .. Stay Safe All BLighters
After trying dipipanone (bloody wonderful, 20mg rectally
izo
Bluelighter
After trying dipipanone (bloody wonderful, 20mg rectally) and reading that replacing the piperidine with a morpholine ring produces identical effects, norphenadoxone seems like it would be wonderful (as well as legal everywhere, as far as I can tell!)
sadly missed dipiapanone, is it rather similar to methadone. tried the desmethylmoramide some weeks ago, it was a total disapointment. somewhat of an opioidergic mu glow in your head for some hours, nothing more. no good bodyload, no sedation. a rather pointless high.
fastandbulbous
Bluelight Crew
Yeah, it seems even a slight deviation from dextromoramide buggers activity. 10mg peach Palfium was very nice...sadly missed dipiapanone, is it rather similar to methadone. tried the desmethylmoramide some weeks ago, it was a total disapointment. somewhat of an opioidergic mu glow in your head for some hours, nothing more. no good bodyload, no sedation. a rather pointless high.
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
I was truly awed that Janssen found dextromoramide since it's an island of activity. The fact the methyl side-chain is moved and that destroyed activity of all other related compounds shows he really was looking at Dreiding models with properly calculated angles.
fastandbulbous
Bluelight Crew
Well, they do run an incredible range of compounds, based on SAR predictions (when I were lad, there was none of this QSAR malarky!I was truly awed that Janssen found dextromoramide since it's an island of activity. The fact the methyl side-chain is moved and that destroyed activity of all other related compounds shows he really was looking at Dreiding models with properly calculated angles.
I think there are other one off, opioids (I think dioxaphetyl butrate is another)
fastandbulbous
Bluelight Crew
3-FPM is also very active, if IMed. Thibg is, most people do not like to fuck around with needles. Can't remember where I mentioned it, but my late wife and myself would IM 3-FPM as an aphrodisiac, that was great. Add in 4-FMPH and it was fuck until I was too sore to wear underpants! (A day around the house wearing bathrobesYou what?
3-fpm when vaped was the fuckin best stimulant I've ever had. I'd love to get hold of the parent compound...
I have mentioned that the meta substituted methylphenidates are the 'creme de la creme', but no one seems to have taken that up.
Phenmetrazine was nice, but I only ever tried it orally: it is supposed to be amazing via 'the needle' (that's Basil Rathbone's final words, in 'Hound of the Baskervilles' - "Watson, the needle". His only reference to Holmes coke habit, in god knows how many films).
The piperazines do seem that they might be very effective, as N-benzylpiperazine is an effective stimulant, so it would combine an amine function on a carbon one carbon atom and two carbon atoms away from the aromatic ring.
If RC firms want a super effective DRI/NRI compound, as well as the ring halogen substitution, they need to replace the piperidine ring of methylphenidate with a morpholine ring.
Apologies if I've already mentioned the above. Three weeks of morphine (oramorph), for wound dressing changes, feels like the first stages of some sort of dementia.
emkee_reinvented
Bluelighter
Palfium is DextroMoramide right. Long before my time and allready out of dr's and junkie's hand's.
I think the above image is of Dutch Palfium. The Dutch use the REALLY simple trick of making it impossible to inject pills because all of their pills are about the size of a paracetamol. I mean, a 500mg paracetamol tablet weighs almost a gram so..... if you have something that big with just 10mg in it.... it cannot be shot; simple idea.
Last I heard about it they raided a medical distribution centre and stole the whole supply. They were probably very pleased they didn't get caught.
But as far as injecting goes, like Tilidine, DextroMoramide is said to produce an amazing rush oral. Equaling Heroine IV-ed. Many junkie's got a script just by saying they intended to quit Heroine. Lots of less bureaucracy or paperwork. When dr's where still naieve human's.
Nas47
Road-Weary Traveler
Still remember the glass bottle-Phendimetrazin bitartrat(think,that is different from phenmetrazin) from my mum's meds.Yellow pills.Never took from them.....cause i got no interest in stims back then in 90-ies....product of DDR....antiobesity pills......after she has gone i gave the bottle to someone
TotalTotalness
Bluelighter
- Joined
- Dec 2, 2012
- Messages
- 22
3-fpm for me was also hit and miss, but I did find it a bit addictive when IV'ed...was a nice rush, but not worth the damage
AlsoTapered
Bluelighter
BTW the first synthetic drug 'epidemic' was Sweden in the 50s. Phenmetrazine was introduced as a [P] medicine i..e. any adult could simply purchase it from any pharmacy. But apparently it produces an amazing rush if injected and soon their was estimated to be close to 100,000 injecting users. By 1956 it became a [POM] but even then most of it was still feeding the needle so in 1958 it was banned.
We made a sample of G-130 and by all accounts it was unremarkable. But 1 person tried IVing it and was instantly singing it's praises and begging to get more.
It's rare but not unique for psychoactives to produce significantly different effects if injected. I can only think of Diconal (dipipanone + cyclizine) and piritramide although it DOES appear that many of the diphenylheptanone opioids, especially if mixed with a first generation antihistamine produce a spectacular 'flash'.
I have no idea if the ring-substituted derivatives of phenmetrazine would behave the same way but the Swedish experience was totally unexpected. For a few years phenmetrazine remained a [P] in some other nations and so Sweden's first significant drug smuggling problem was the illegal importation of phenmetrazine.
Someone mentioned fencamfamine. Yes, it's certainly better than camfentamine. It's very subtle unless, like phenmetrazine, it's injected. Then the effects are said to be pretty impressive.
I've never enjoyed the rush/flash of any drug... apart from maybe DMT but it's arguable that the entire experience is a rush. But for some people, it's those initial effects; the dynamic changes in brain-chemistry that they really crave. Those people never seem to last long because one has to be intent on self-destruction to keep on pushing back against tolerance.
We made a sample of G-130 and by all accounts it was unremarkable. But 1 person tried IVing it and was instantly singing it's praises and begging to get more.
It's rare but not unique for psychoactives to produce significantly different effects if injected. I can only think of Diconal (dipipanone + cyclizine) and piritramide although it DOES appear that many of the diphenylheptanone opioids, especially if mixed with a first generation antihistamine produce a spectacular 'flash'.
I have no idea if the ring-substituted derivatives of phenmetrazine would behave the same way but the Swedish experience was totally unexpected. For a few years phenmetrazine remained a [P] in some other nations and so Sweden's first significant drug smuggling problem was the illegal importation of phenmetrazine.
Someone mentioned fencamfamine. Yes, it's certainly better than camfentamine. It's very subtle unless, like phenmetrazine, it's injected. Then the effects are said to be pretty impressive.
I've never enjoyed the rush/flash of any drug... apart from maybe DMT but it's arguable that the entire experience is a rush. But for some people, it's those initial effects; the dynamic changes in brain-chemistry that they really crave. Those people never seem to last long because one has to be intent on self-destruction to keep on pushing back against tolerance.



