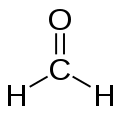
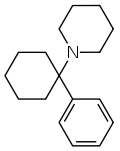
Gaz_hmmmm
Bluelighter
I was looking at this molecule and looking at GHB and 1.4 - Butanediol and thinking surely it'd be easy to synthesise G' from this stuff?
But that's another thread!
My point is... if you had this stuff would it have a 1.4B/GBL effect and turn into GHB in the body or even turn into 1.4B?
NO I DO NOT HAVE ANY n-BUTANOL / n-BUTYL ALCOHOL AND AM NOT GOING TO TRY IT!
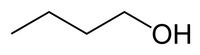
N-Butanol / n-butyl alcohol
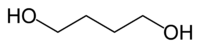
1.4-Butanediol
GHB acid (As in not Na-GHB)
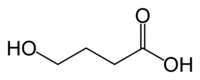
So what do you guy's think?
The new 1.4B/GBL or just a chemist's precursor to GHB?
But that's another thread!
My point is... if you had this stuff would it have a 1.4B/GBL effect and turn into GHB in the body or even turn into 1.4B?
NO I DO NOT HAVE ANY n-BUTANOL / n-BUTYL ALCOHOL AND AM NOT GOING TO TRY IT!
N-Butanol / n-butyl alcohol

1.4-Butanediol

GHB acid (As in not Na-GHB)

So what do you guy's think?
The new 1.4B/GBL or just a chemist's precursor to GHB?