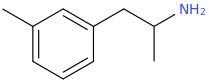
None of the indoles or pseudoindolws will work.
Aromatics are important for binding. Not only are they planer & lipophilic but they also have pi bonds. People have made meth with cyclohexane replacing benzene - MUCH less active. If you tried it with MDMA, the MD ring wouldn't be planer.
Others have been made.
9EP4MV3Url | C11H14BrNO2 | CID 156337249 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.

pubchem.ncbi.nlm.nih.gov
PubChem is the world's largest collection of freely accessible chemical information. Search chemicals by name, molecular formula, structure, and other identifiers. Find chemical and physical properties, biological activities, safety and toxicity information, patents, literature citations and more.

pubchem.ncbi.nlm.nih.gov
Please check PubChem. It's free, takes a moment, avoids people declaring that they have 'invented' a new compound (in spite of having no idea how to make it( and most importantly because if you WANT to get better, reading all of the papers and references on PubChem is the way to go.
I'm always willing to help people improve their knowledgebase, but It's a labour to have to constantly correct when the answers are already on a free online database.
BTW I've been begging BL to support SMILES format so we can go IUPAC<->SMILES<->Image instantly. A HUGE benefit to us all.