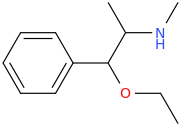
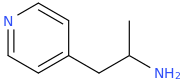
The joke about
Phenylephrine in the circles who actually formulate OTC medicines is that it's totally INactive. They just needed SOMETHING to pretend that was they were selling WORKED... which it doesn't. So do not be surprised that they offered no proof... only proof that it wasn't (too) toxic.
Ah, the good old Wacker Oxidation.
If you can form the imine by actively removing the water so that the amine + ketone <---> imine becomes amine + ketone ----> imine, NaBH4 will work perfectly well. It was presumed that the milder NaBH3CN would produce the highest yield. It does not. It's slower as you have to slowly add the NaBH4 but works fine.
Oh, and a US friend informed me that while NH2CH3, NH2CH3.HCl, NH2CH3.H2SO4 & NH2CH3.H3PO4 are all automatically 'caught' by the software of supply houses as is NH2CH3.H2CO3.....
(NH2CH3)2.H2CO3 (methylammonium carbonate) is (or at least was) NOT so they made a LOT of $$$ LEGALLY not by making MDMA but by simply selling on the above at triple the price they paid.
I might also add that if one uses an addition salt of methylamine, it's a solid. So I SUSPECT the old trick of using a calculated amount of NaOH.H2Othen one can freebase the amine and go on to remove the water (the NaOH forming higher hydrates) and just add NaBH4...
I've not tried it, but it appears a reasonable solution.
What I AM keen to find is the direct route from the glycidate salt of MP-P2P directly to MDA. I keep on hearing whispers and I know that MDA powder is now abundant in some European nations. In the book 'E for Ecstasy' the author notes that their IS a route but whoever worked it out is keeping an iron grip on it because it means MDA production costs drop significantly...
You may laugh but in The Netherlands, decentralization of MD(M)A production has proved a successful technique for eluding the UDS. Instead of 1 BIG lab making 30-50Kg batches, now a lot of it is students who just make hundreds of grams at a time. They sell it to guys who operate presses.
BTW SGOTI makes a good point. You would be amazed at how cheaply Chinese supply houses will provide you with immediate precursors. The synthesis of U47700 was:
Image rs hosted in ImgBB

ibb.co
That's right. 1 step, room-temperature, quantitative yield.
And U5 is some x3 more potent.... and JUST as simple.
It wasn't some chemistry god who stepped down from a mountain and decreed that a totally novel structure would in fact be some x7.5M with a duration of 6 hours. It was the result of reading THOUSANDS of papers and patents, an intensive series of E-mails where various suppliers were provided with the references for the synthesis of the diamine and the resources required (time, equipment, reagents & solvents) including links to OTHER Chinese suppliers giving the calculated cost.
That done, the winner simply sent the diamine correctly labelled and appropriately packaged to a UK business address.
Then the trained monkeys we had doing those final steps (who ALSO got the references and costings) produced it.
The disaster was that I had INSISTED that it was provided in the form of 5mg tablets. My boss at the time decided to sell on the bulk powder, I had a breakdown and quit. I was doing MY best to ensure NOBODY was put in harms way BUT to save the £1200 to have it tabletted... well, you can read the news at the time.
But no genius was involved - a lot of hard work was employed. Their IS no substitute for hard work. Not in medicinal chemistry, not in ANY job. It's a willingness to potentially waste hundreds of hours of effort for nothing (like us making AH-7921 but deciding it wasn't any good and people were taking more and more in an attempt to MAKE it do anything except to stop abstinence syndrome. For that, I believe it really does have medical utility since none of US could feel any positive effects BUT dependant users found it relieved symptoms....
But that's another story.
As a rule of thumb, an RC should be just 1 synthetic step from the legal precursors bought openly on the market. 2 & even 3 steps are possible. Diphenidine was 1 step, MDAR was 2 steps, diclazepamwas 2 steps, pyrazolam was 3 steps. Nothing we EVER more than 3 steps, involved the use of highly toxic (or otherwise hazardous compounds) and the total yield was never lower than 76%.
So you can sit there inventing all you want, but the people who ACTUALLY succeed look for simplicity, low cost and safety above all else. I've survived a lab explosion in Maarsseveen (just outside Utrecht in The Netherlands) and to be frank, it was our own fault. In our favour, 2CI was NOT legally controlled in The Netherlands at the time and as my lawyer pointed out 'after how badly you were all injured, a Dutch judge would consider you to have paid the penalty with interest'.
This is called EXPERIENCE and you won't find it on a Wiki page. Still, considering I began in 1988 and haven't even been arrested let alone charged, even THAT clusterf*ck was entered into knowing most of the risks we faced.
What DID I learn? Don't use a garage with a combi-boiler in it as a lab. As soon as someone ran hot water to was their hands, the toluene vapour went up taking off the roof. Not an experience I would wish anyone to go through. So learn from MY mistakes.
Technically we are not supposed to discuss synthesis but since everything I say is in MULTIPLE places on the internet, I don't quite get the point.
In future, simply produce word documents and simply place links to them on the site. After all, we link to references all the time and nobody ever mentions it. All we have done is reprinted what is elsewhere so, IMO, a link to your OWN documents should be fine.