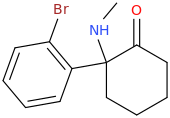
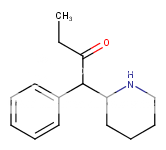
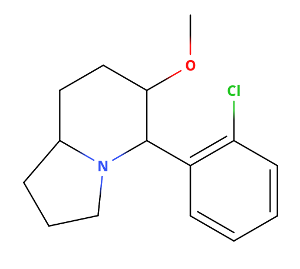
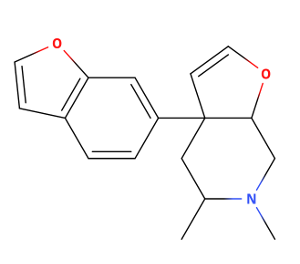
What do you base the notion that U-47700 is an SNDRI on? ......
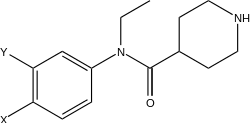
This series of compounds from this danish patent (the structure at the bottom, cf the wiki to link up). They are extremely potent SNDRI but with more NDRI selectivety (Ki-SERT = 0.37uM; Ki-DA = 0.021uM and Ki-NE = 0.0097uM) about 12,000 more potent than cocaine as NDRIs. Compare the structure to that of U4x
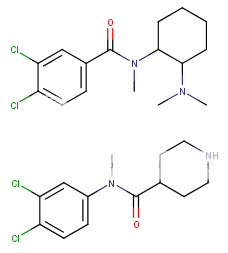
Now that i have time to go through that patent again, actually the di-Chloro substitution pattern doesn't skew selectivety towards SERT as with cocaine and MPH analogs where 3,4d-Cl2phenyl or naphthyl increase SERT dramatically. That's why I thought it may be more serotonergic. But obvioulsy not. So nothing to worry about serotonergic toxicity. Assuming binding similar in both cases, it will be rather selective NDRI and not SNDRI (at least by a factor of10x !)
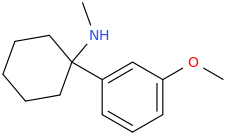
Notice the inverted amide and the positively charged dimethylamino on the cyclohexyl replaced by a piperidine. For transporters it doens't really matter a lot whether or not a N+ is present (it only reduce activity by 100x at most, which is still huge going say from 0.025uM to 2uM still 150x times higher than cocaine!! .. The structural, steric shape of the molecule is most critical .. but who knows until tested?
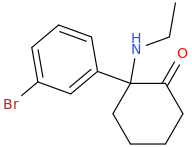
The Tramadol-like NDRI (if any as I assumed) may explain why the U4x are potentially extremely addictive : extremely potent NDRI + mu Opioid agonist= speed-ball without serotonergic. Which paradoxically will make them safer since deadly opiates-induced respiratory depression is balanced out by stim NE activity (correct me if I am wrong.
or better yet the methoxy that can potentially get metabolized into a phenol .. I dont think they been tested: the original patent focus more on kappa selective rather that mu so they didnt really bother wasitng time on U4x!!The phenol does seem like a good idea though, has it been tested?