-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
neurotic
Bluelighter
hey Nagelfar if you're serious about learning about this shit and don't wanna have to look for information scattered around everywhere on the internet (tiresome), i'd suggest you Khan Academy's organic chemistry course. it's pretty neat. if you want to go a little bit deeper / put some more effort on it too, then you can pick up an introductory book on organic chemistry such as Janice's one. you can find a PDF of it online. considering the amount of time you spend on this subject (drugs) i'd think it'd be a great thing for you to do, very rewarding: the more chapters you read, the deeper becomes your understanding and a lot of things you see start to make more and more sense. it's just so cool. it'll give you a more solid basis for your molecular ideas.
quick unrelated question: can benzoic acid crystals look like little tiny white needles?
quick unrelated question: can benzoic acid crystals look like little tiny white needles?
^ Benzoic acid often crystallises to be colourless needles. As does very similar compounds - even aspirin can be fine colourless needles.
Dresden, they may be active but will be very rapidly metabolised and aldehydes are typically avoided because it's so reactive. In acidic conditions it's going to go one of two ways - Best case: disintegrate into CO2 and H2O, Worst case: The carbonyl carbon will be an easy site for nucleophiles.
The first has the fragile N-C-O moiety people were discussing earlier
Dresden, they may be active but will be very rapidly metabolised and aldehydes are typically avoided because it's so reactive. In acidic conditions it's going to go one of two ways - Best case: disintegrate into CO2 and H2O, Worst case: The carbonyl carbon will be an easy site for nucleophiles.
The first has the fragile N-C-O moiety people were discussing earlier
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
The first has the very stable R-(C=O)-NH-R' amide functional group which is found all over the place, most notably in proteins and peptides. People were discussing aliphatic N-C-O's earlier. I thought amides were so commonly known to be stable not to need mention in the earlier discussion.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
The other molecule is remedied easily enough by this:

Amphetamine's logP is already low enough.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Does that stop ephedrine?
It's probably one of the reasons why ephedrine is required in higher molar concentrations than amphetamine, and why it has so much peripheral action but really not that much central activity.
Psychedelic Indoles alkaloids: Ibogaine:

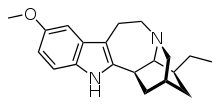
Design simpler :

would that retain MUCH of ibogaine activity (5HT2a/2c and 5HT3 agonist, mu, kappa, sigma opioid agonist, NMDA antagonsism and DAT inhibition among others?
Ibogaine is a psychedelic.[12] The experience of Ibogaine is broken down in two phases, the visionary phase and the introspection phase. The visionary phase has been described as oneirogenic, referring to the dreamlike nature of its psychedelic effects, and lasts for 4 to 6 hours. The second phase, the introspection phase, is responsible for the psychotherapeutic effects. It can allow people to conquer their fears and negative emotions. Ibogaine catalyzes an altered state of consciousness reminiscent of dreaming while fully conscious and aware so that memories, life experiences, and issues of trauma can be processed.[13]


Design simpler :

would that retain MUCH of ibogaine activity (5HT2a/2c and 5HT3 agonist, mu, kappa, sigma opioid agonist, NMDA antagonsism and DAT inhibition among others?
disclaimer: wiki
As of 2009, ibogaine is unregulated in Canada[56][57] and Mexico.[5]
As of 2015 in the United Kingdom, ibogaine is not listed under the Misuse of Drugs Act and so is legal to possess, however distribution is illegal.[58][59]
Ibogaine is schedule I in Sweden.[60]
Ibogaine is classified as a Schedule I-controlled substance in the United States,[61] and is not approved there for addiction treatment (or any other therapeutic use) because of its hallucinogenic, neurotoxic, and cardiovascular side effects, as well as the scarcity of safety and efficacy data in human subjects.[62]
Ibogaine is illegal in Norway (as are all tryptamine derivatives).[63]
Ibogaine is unregulated in Germany, but for medical use it can be regulated by the pharmacy rules (AMG).
Ibogaine was gazetted in New Zealand in 2009 as a non-approved prescription medicine.[64]
On January 14, 2016, Ibogaine was legalized for prescription use in São Paulo, Brazil, with this legalization to extend to the rest of the country in a few months.[14]
In most other countries it remains unregulated and unlicensed.[65][66]
Incunabula
Bluelighter
- Joined
- Dec 10, 2010
- Messages
- 1,861
I always wondered why there weren't any more ibogaine analogues. It's a very interesting area. I know some analogues have been developed, but they were made with a focus on the anti-addictive potential of ibogaine, and not the psychedelic.
Edit:
This one would be interesting to sample. If it was possible to synthesize, that is.

Edit:
This one would be interesting to sample. If it was possible to synthesize, that is.

Last edited:
roi
Bluelighter
- Joined
- Sep 2, 2013
- Messages
- 1,545
If it was possible to synthesize, that is.
Should be quite easy using phosphorus pentasulfide.
Bagseed
Bluelighter
some people here always say that N-C-O in a molecule tends to be unstable. I cannot seem to find the reason why, will sombody enlighten me? 
Incunabula
Bluelighter
- Joined
- Dec 10, 2010
- Messages
- 1,861
Oops, I just realised that I put an oxygen instead of nitrogen in the indole (5-MeO-DIBF style). It wasn't a mistake, just drew it a while ago, and missed that it was two ideas in one. Not that either idea was very original.
 cool.
cool.
OkayShould be quite easy using phosphorus pentasulfide.
some people here always say that N-C-O in a molecule tends to be unstable. I cannot seem to find the reason why, will sombody enlighten me?
That REALLY depends on the context..
I think iirc what made someone or some people say something like that is when you e.g. have cyclic or polycyclic molecules and there is an N-C-O present but in particular in a R-C(NR'2)(OR")-R''' pattern called a hemiaminal ether.

Like acetals, those are 'unstable' in that they exist not only in ring formation but also a ring-opened 'chain' formation. Carbohydrates (sugars) do that, too: they flop open and closed very fast and those states are in an equilibrium.
In the case of hemiaminals it's worse cause under a lot of conditions it collapses to the imine.
Last edited:
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Psychedelic Indoles alkaloids: Ibogaine:


Design simpler :

would that retain MUCH of ibogaine activity (5HT2a/2c and 5HT3 agonist, mu, kappa, sigma opioid agonist, NMDA antagonsism and DAT inhibition among others?
I would say not really. The thing separating ibogaine from say 5-Meo-DMT is of course the cyclic system and unconstraining the molecule as you have done results in the cyclohexane taken much out of its original plane. I'd say this would effect binding affinities of its many targets to an appreciable degree.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Should be quite easy using phosphorus pentasulfide.
It would be very hard due to integrating the benzofuran system into the molecule. You would probably have to do it this way, but of course replace the indole with the benzofuran:
https://www.youtube.com/watch?v=QcHxjckH0DE
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
some people here always say that N-C-O in a molecule tends to be unstable. I cannot seem to find the reason why, will sombody enlighten me?
A non-amide N-C-O is unstable for the same reason that acetals are unstable, as said above. The 2 substituents on the carbon pull electron density from it and make it susceptible to nucleophilic attack by even weak nucleophiles like water. The only way they would be stable is if they are cyclic (entropic stability) or if the carbon is sterically protected from nucleophiles.
- Status
- Not open for further replies.






