-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Nagelfar
Bluelight Crew
Source?
Fused tropane-derivatives as neurotransmitter reuptake inhibitors US PATENT 5998405 A

^the last one has picomolar affinity for SERT, the others have 1.6 & 2.3, respectively (cf. cocaine's 155)

^many of these have picomolar affinity at DAT, NET *and* SERT
Last edited:

[h=2]Abstract[/h]N,N-Diallyltryptamine (DALT) and 5-methoxy-N,N-diallyltryptamine (5-MeO-DALT) are two tryptamines synthesized and tested by Alexander Shulgin. In self-experiments, 5-MeO-DALT was reported to be psychoactive in the 12–20 mg range, while the unsubstituted compound DALT had few discernible effects in the 42–80 mg range. Recently, 5-MeO-DALT has been used in nonmedical settings for its psychoactive effects, but these effects have been poorly characterized and little is known of its pharmacological properties. We extended the work of Shulgin by synthesizing additional 5-substituted-DALTs. We then compared them to DALT and 5-MeO-DALT for their binding affinities at 45 cloned receptors and transporter proteins. Based on in vitro binding affinity, we identified 27 potential receptor targets for the 5-substituted-DALT compounds. Five of the DALT compounds had affinity in the 10–80 nM range for serotonin 5-HT1A and 5-HT2B receptors, while the affinity of DALT itself at 5-HT1A receptors was slightly lower at 100 nM. Among the 5-HT2 subtypes, the weakest affinity was at 5-HT2A receptors, spanning 250–730 nM. Five of the DALT compounds had affinity in the 50–400 nM range for serotonin 5-HT1D, 5-HT6, and 5-HT7 receptors; again, it was the unsubstituted DALT that had the weakest affinity at all three subtypes. The test drugs had even weaker affinity for 5-HT1B, 5-HT1E, and 5-HT5A subtypes and little or no affinity for the 5-HT3 subtype. These compounds also had generally nanomolar affinities for adrenergic α2A, α2B, and α2C receptors, sigma receptors σ1 and σ2, histamine H1 receptors, and norepinephrine and serotonin uptake transporters. They also bound to other targets in the nanomolar-to-low micromolar range. Based on these binding results, it is likely that multiple serotonin receptors, as well as several nonserotonergic sites are important for the psychoactive effects of DALT drugs. To learn whether any quantitative structure–affinity relationships existed, we evaluated correlations among physicochemical properties of the congeneric 5-substituted-DALT compounds. The descriptors included electronic (σp), hydrophobic (π), and steric (CMR) parameters. The binding affinity at 5-HT1A, 5-HT1D, 5-HT7, and κ opioid receptors was positively correlated with the steric volume parameter CMR. At α2A, α2B, and α2C receptors, and at the histamine H1 receptor, binding affinity was correlated with the Hammett substituent parameter σp; higher affinity was associated with larger σp values. At the σ2 receptor, higher affinity was correlated with increasing π. These correlations should aid in the development of more potent and selective drugs within this family of compounds.
"Receptor binding profiles and quantitative structure–affinity relationships of some 5-substituted-N,N-diallyltryptamines"
Nicholas V. Cozzia, b, Paul F. Dale
Bioorganic & Medicinal Chemistry Letters
Volume 26, Issue 3, 1 February 2016, Pages 959–964
http://www.sciencedirect.com/science/article/pii/S0960894X1530367X ($paying$ article) enjoy the read!
Nagelfar
Bluelight Crew

≡

I love my cubism:
protonate it, and be able to front and back bridge the tropane from the same nitrogen starting point (don't have to split off from one methylene unit away and possibly skew the optimum factors constraining the nitrogen)
i.e. (Scheel-Krüger et al. U.S. Patent 5,998,405 compound #1 + S. Singh's cocaine antagonist paper's compound # 131a)

≡

Last edited:
Nagelfar
Bluelight Crew
Raihiar
Greenlighter
- Joined
- Feb 1, 2011
- Messages
- 43
thanx a million
oh yaaaaay!
I LOVE you for this since i've been a huge fan of 5-meo-dalt (when it was still quasi-legal and relatively easly acquired here )
)
binged on it - the only chemical i've ever vaped and generally had an amazing time with it, and all that in spite of the fact that many 'normal' psychedelics seem to overwhelm me and turn bad eventually.
maybe some promising ones hit the market, and i might try my luck once again
And since i dont have anything picture-wise to show: behold the 1-minute-paint cyclomethane


"Receptor binding profiles and quantitative structure–affinity relationships of some 5-substituted-N,N-diallyltryptamines"
Nicholas V. Cozzia, b, Paul F. Dale
Bioorganic & Medicinal Chemistry Letters
Volume 26, Issue 3, 1 February 2016, Pages 959–964
http://www.sciencedirect.com/science/article/pii/S0960894X1530367X ($paying$ article) enjoy the read!
oh yaaaaay!
I LOVE you for this since i've been a huge fan of 5-meo-dalt (when it was still quasi-legal and relatively easly acquired here
binged on it - the only chemical i've ever vaped and generally had an amazing time with it, and all that in spite of the fact that many 'normal' psychedelics seem to overwhelm me and turn bad eventually.
maybe some promising ones hit the market, and i might try my luck once again
And since i dont have anything picture-wise to show: behold the 1-minute-paint cyclomethane

Nagelfar
Bluelight Crew

phenmetrazine inspired.
(You know its unnatural, when the structure is small, and it's name gets rendered like: 6-phenyl-1λ⁶,2λ⁶-dithia-4-azacyclohexa-1(6),2,4-trien-1-yne ;-j )

(3-phenylpyridine, there we go... Normalcy.)
And because what's a contribution of mine if there isn't a cocaine derivative in it?:

Last edited:
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
All the low hanging fruit has now been harvested. With the hundreds and hundreds of new drugs which flooded the RC market at some point in the last couple of years, the only two RCs notable enough to become street drugs were

MEPHEDRONE
and

MXE.
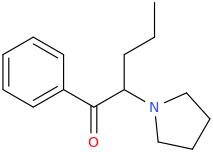
aPVP would have made the cut, except other stimulants work just as well as it and therefore it represented no real improvement over what was already available.
WILL ALL THE CHEMISTS GO BACK TO FLOODING THE MARKET WITH MDMA LIKE IT IS FLOODED WITH METH RIGHT NOW; LET'S KEEP THE METH TOO! NOBODY WANTS RCS ANYMORE
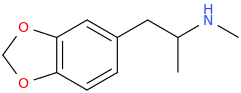
MDMA
On the subject of meth (my favorite), I just want to thank God that that the subversive Mexican cartels are giving America a middle finger by flooding our street markets with good meth. WAKE UP PEOPLE. (literally)
METHAMPHETAMINE
And Nagelfar, I'm not trying to be rude, but that thing you drew right there is absurd to the point of fatuity from a chemical or pharmacological viewpoint and looks like an impossible train wreck.

MEPHEDRONE
and

MXE.

aPVP would have made the cut, except other stimulants work just as well as it and therefore it represented no real improvement over what was already available.
WILL ALL THE CHEMISTS GO BACK TO FLOODING THE MARKET WITH MDMA LIKE IT IS FLOODED WITH METH RIGHT NOW; LET'S KEEP THE METH TOO! NOBODY WANTS RCS ANYMORE

MDMA
On the subject of meth (my favorite), I just want to thank God that that the subversive Mexican cartels are giving America a middle finger by flooding our street markets with good meth. WAKE UP PEOPLE. (literally)

METHAMPHETAMINE
And Nagelfar, I'm not trying to be rude, but that thing you drew right there is absurd to the point of fatuity from a chemical or pharmacological viewpoint and looks like an impossible train wreck.
Last edited:
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Consider methylphenidate:

Here are a few rigidified molecules which would help to uncover the binding roles of the alkyl esters in the methylphenidate series:
First, we consider this rigidified molecule:

Then:

Acetal hydrolysis is obviously a concern with the second molecule (the first one also has N-C-O which could act like an acetal), however this shouldn't be a problem if evaluated in vitro. The more potent molecule should suggest to which angle the alkyl group in methylphenidate is oriented.
The following molecule is simply rigidified methylphenidate but the pyridine ring is flipped (I doubt this compound would act effectively because I don't think the nitrogen is in the right place, but just to confirm anyway):

Now, look at some compounds with the ester switched for an amide. The non-basic nitrogen can't really function as a H-bond acceptor like the oxygen (amide double bond character). The carbonyl oxygen is now a better H-bond acceptor though, so we can see where the H-bond acceptor regions are:

Flipped amide:

Once the optimal position for the carbonyl oxygen and alkyl ester has been determined, the ester can be changed to a ketone derivative, and the alkyl chain can be expanded:

These molecules corresponds to ethylphenidate, depending on where the alkyl chain seems to fit best (which would be determined from the above molecules)


I understand the first of these molecules would result in the basic nitrogen possibly being quite hindered from binding to its residue.
The next one corresponds to isopropylphenidate, and they also block the labile carbon from metabolism:

Finally one to push the possible limits of this pocket:


Here are a few rigidified molecules which would help to uncover the binding roles of the alkyl esters in the methylphenidate series:
First, we consider this rigidified molecule:

Then:

Acetal hydrolysis is obviously a concern with the second molecule (the first one also has N-C-O which could act like an acetal), however this shouldn't be a problem if evaluated in vitro. The more potent molecule should suggest to which angle the alkyl group in methylphenidate is oriented.
The following molecule is simply rigidified methylphenidate but the pyridine ring is flipped (I doubt this compound would act effectively because I don't think the nitrogen is in the right place, but just to confirm anyway):

Now, look at some compounds with the ester switched for an amide. The non-basic nitrogen can't really function as a H-bond acceptor like the oxygen (amide double bond character). The carbonyl oxygen is now a better H-bond acceptor though, so we can see where the H-bond acceptor regions are:

Flipped amide:

Once the optimal position for the carbonyl oxygen and alkyl ester has been determined, the ester can be changed to a ketone derivative, and the alkyl chain can be expanded:

These molecules corresponds to ethylphenidate, depending on where the alkyl chain seems to fit best (which would be determined from the above molecules)


I understand the first of these molecules would result in the basic nitrogen possibly being quite hindered from binding to its residue.
The next one corresponds to isopropylphenidate, and they also block the labile carbon from metabolism:

Finally one to push the possible limits of this pocket:

Last edited:
Nagelfar
Bluelight Crew
All the low hanging fruit has now been harvested. With the hundreds and hundreds of new drugs which flooded the RC market at some point in the last couple of years, the only two RCs notable enough to become street drugs were
The MPH analogs too, don't forget. I did ethylphenidate, it was viable.
And Nagelfar, I'm not trying to be rude, but that thing you drew right there is absurd to the point of fatuity from a chemical or pharmacological viewpoint and looks like an impossible train wreck.
Compliment accepted.

phenmetrazine inspired.
(You know its unnatural, when the structure is small, and it's name gets rendered like: 6-phenyl-1λ⁶,2λ⁶-dithia-4-azacyclohexa-1(6),2,4-trien-1-yne ;-j )

(3-phenylpyridine, there we go... Normalcy.)
And because what's a contribution of mine if there isn't a cocaine derivative in it?:

what do you call that last tropane?.. does it smell??? not sure about those
But I like pyridines
I like pyridines..now:

about twice as potent as cocaine as DAT reuptake inhibitor.. pure and clean DRI no SERT or NET.. now add a Nitrogen like this

20x Cocaine (my bet!) nice and clean DRI nice logP .. one more N like this:

200x cocaine? (my bet) but logP start becoming shitty (1.8 still decent..)
Or change 1 phenyl with an indole like this:

nice clogP= 3.3 too bulky for DAT but SERT might like it.. now append a dimethylaminoethyl like this ..et voila

1DP-DMT ("Fat Mao"

about twice as potent as cocaine as DAT reuptake inhibitor.. pure and clean DRI no SERT or NET.. now add a Nitrogen like this

20x Cocaine (my bet!) nice and clean DRI nice logP .. one more N like this:

200x cocaine? (my bet) but logP start becoming shitty (1.8 still decent..)
Or change 1 phenyl with an indole like this:

nice clogP= 3.3 too bulky for DAT but SERT might like it.. now append a dimethylaminoethyl like this ..et voila

1DP-DMT ("Fat Mao"
This might've already popped up in this thread, but have 3-alkylaminobenzothiophene derivatives (indole bioisostere) been suggested? The sulphur could be a good metabolic handle to decrease half life.


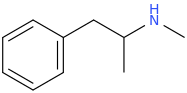
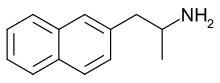
isn't thiophene isostere closer to naphthalene than indole? 2-naphthyl isopropyl amine is empathogen with binding profile very much similar to MDMAs (but 10x more potent).
- Status
- Not open for further replies.




















