Must be your screen resolution then...
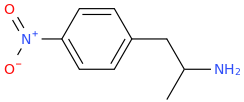
I don't know about that 'getting high' event but the structure would be what Shulgin called A for amylescaline. Not pentescaline since P is used for proscaline. It's in the B / buscaline entry. Not that promising?
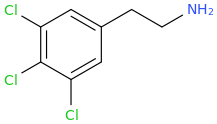
Personally I'd be curious what is beyond MAL? Say isobuscaline or even neopentescaline..
Then again the thio's or haloalkyls could be just as promising - like say a 2C-T-7 (fluoropropylthio) analogue?
I don't know about that 'getting high' event but the structure would be what Shulgin called A for amylescaline. Not pentescaline since P is used for proscaline. It's in the B / buscaline entry. Not that promising?
Personally I'd be curious what is beyond MAL? Say isobuscaline or even neopentescaline..
Then again the thio's or haloalkyls could be just as promising - like say a 2C-T-7 (fluoropropylthio) analogue?






![(6aR,10aR)-6,6,9-trimethyl-3-propylthio-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol.png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%286aR%2C10aR%29-6%2C6%2C9-trimethyl-3-propylthio-6a%2C7%2C8%2C10a-tetrahydro-6H-benzo%5Bc%5Dchromen-1-ol.png&hash=7580b25d3eec7d442278562dba670bae)