Nagelfar
Bluelight Crew
roi, your last contribution is interesting too. You don't see many cyclopropanes on monoaminergic-type drugs, but it's small enough you'd think it could work in some capacity.

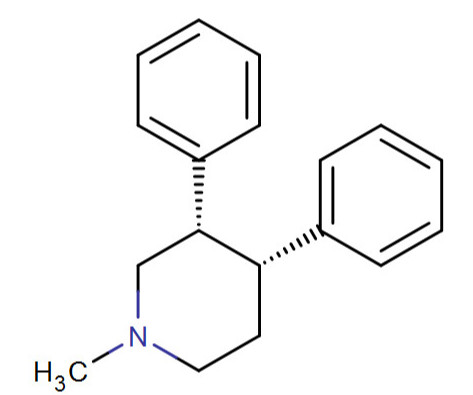
The above is another one of my Super-Cocaines; this time based upon the benzoylthiomethylecgonine (but of course, not a benzoyl or methyl in this instance but augmented from Singh's 223f cocaine analogue for the phenyl branch + 219e's modification on the nitrogen and 200 replacing the carbmethoxy and 225c on the "6/7" tropane substitution position)

The above is another one of my Super-Cocaines; this time based upon the benzoylthiomethylecgonine (but of course, not a benzoyl or methyl in this instance but augmented from Singh's 223f cocaine analogue for the phenyl branch + 219e's modification on the nitrogen and 200 replacing the carbmethoxy and 225c on the "6/7" tropane substitution position)
Last edited: