sekio
Bluelight Crew
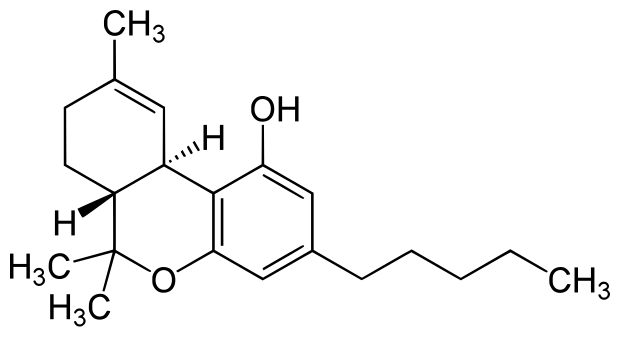
"Myrcene analogs" have lovely scents (geraniol, nerol, linalool etc), but aren't drugs as far as I know. The only reason myrcene crosses the BBB is because it's essentially turpentine, it's super fat soluble... doesn't mean it has any strong activity.
Like others have said, most drug discovery in this day and age is directed, not purely serendipity-based. The "hit rate" for coming up with workable drugs for a particular need from random atom-smashing is pretty much nil. That's not to say it doesn't happen, but I think you need to clarify what exactly you're trying to say here. "I have a hunch that compounds derived from XYZ will be active some way" is not really how this works and isn't ground for running around shouting Eureka.
Like others have said, most drug discovery in this day and age is directed, not purely serendipity-based. The "hit rate" for coming up with workable drugs for a particular need from random atom-smashing is pretty much nil. That's not to say it doesn't happen, but I think you need to clarify what exactly you're trying to say here. "I have a hunch that compounds derived from XYZ will be active some way" is not really how this works and isn't ground for running around shouting Eureka.