SeenSoFar
Bluelighter
- Joined
- Jun 21, 2013
- Messages
- 241

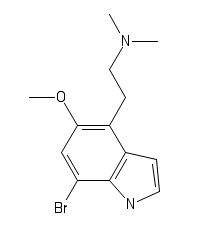
4-HO-MMT + some other effects like heroin/morphine & 4-AcO-DMT/4-HO-DMT. Was thinking of doing it with the DMT but not sure how that would work. 4 covalents (one coordinate) so N+ ion, plus a negative ion, chlorine or something? Or have a trimethyl on the Nitrogen, keep the diester as an o- and have electrostatic attraction? But yeah, just something fun I made up. Or maybe just a carbonate ion joining both?
***THE FOLLOWING IS HUMOR ONLY AND NOTHING BUT COMEDY HAS BEEN TAKEN INTO ACCOUNT IN MY DESIGN***
Imagine if you will that your compound was to have a tryst with LSD and produce a bastard child... A bastard child that was afflicted with the "LSD-induced genetic damage" that we were told to fear once upon a time... It might look a little bit like this...

Don't forget, it has a methylenedioxy ring, that means it MUST be chock full of AWESOME!
EDIT: Just for the record Mr. Porter, I'm just using your compound as a jumping-off point for some silly comedy. I'm not trying to make fun of you or your design. In fact, I find it somewhat intriguing, and while I have absolutely no idea if it would be active, I do enjoy looking at it.
EDIT 2: Is it just me or does the MDO ring stacked on the pyrrole ring look like a man with a sharp jaw wearing a pope hat and cocking his head?
Last edited: