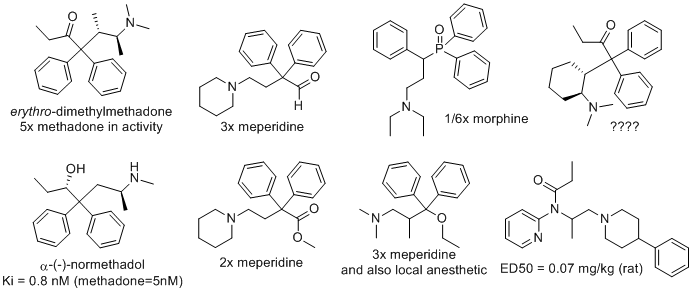
polymath
Bluelight Crew
^ Yeah, that's how someone would end up trying to rob a box of fentanyl patches or eat 500 pills of Imodium in a couple of days when they try to get out of that situation. And even then it would be difficult to balance the amount of opioid and antagonist in their body to not OD instead. About as demonic as "normal" people see drug use is.