blueberries
Bluelighter
14's a pretty magic spot for opioids. It sems like you just stick anything on there and it's a winner. Case in point; 14-PPOM.
N&PD Moderators: Skorpio | someguyontheinternet
That sweet spot oxygen on those morphinans, has it ever been just given a nitrogen? or a sulfur with crazy direct attachments.

The cinnamic acid ester of oxycodeinone
Do that cinnimate 14 to acetorphne see what happens, that, or nearest in synth., is my imaginary pic contribution: edit the pesky endoetheno bridge by inverting the ring one space removed, (up one side, down other) but who knows what that'd do. New biomimetic synth of morphine anyone?
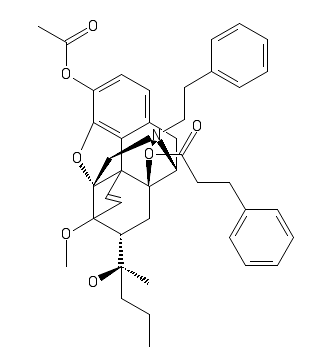
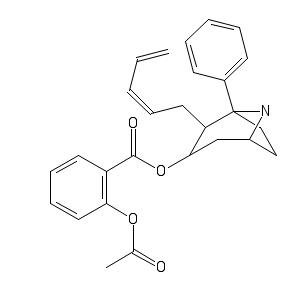
I suspect that it's actually oxycodone cinnamate, i.e. the double bond is reduced.... the same paper that details the other 14-esters has 14-cinnamate as 175 +/- 75 x morphine potency (oxycodone itself at 0.3x).
I was under the impression that codeinone and 14-hydroxycodeinone are crap analgesics.
I believe this is the article you are referring to.I just did a search and found a 1992 findings article on pethidine derivatives made with a tropane substitution, I can't figure how to copy words and addresses with my phone so if someone has access to the article (top of Google search nearly for me) would someone so willing post one or two here for me?
The words "pethidine", "opioid" and "tropane" seem to work
Thank you sekioAnthraxiton is brand name for sodium salt of diclofenac which is shown
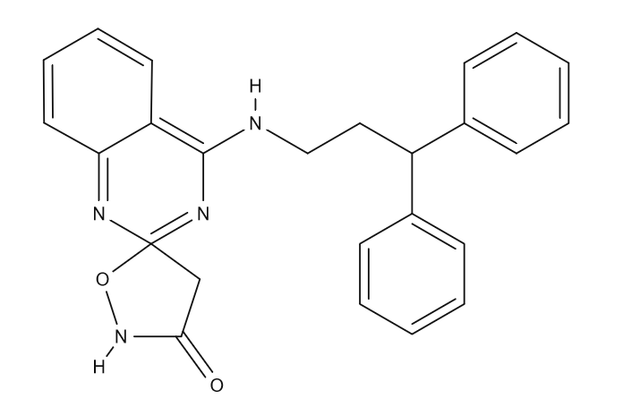
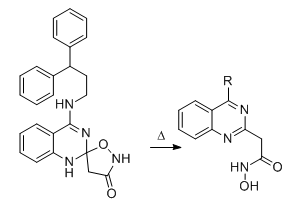
The middle arene beside the penta-spiro and opposite other aryl? That what it's called? Yeah just now wishing I could rotate the faux pi-symmetry cake walk / Chinese firedrill style one carbon around.That has a Texas carbon.