-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Last edited:
I'd be interested in evaluating an azido (Nsub3) group in place of the main ring system chlorine. I've seen nitro. Another EWG that is of interest to me is CFsub3. I'm sure they've all been made & evaluated. Too potent or toxic?
Organic azides do not tend to be the most stable compounds, to the point where inorganic azide salts and low-molecular weight organic azides tend to be shock-sensitive explosives.... not to mention azide salts as well as several organic azides being fairly toxic.
Trifluoromethyl groups shouldn't pose any such problem. I know of trifluoromethyl analogues of nordazepam (triflunordazepam) and clobazam (triflubazam), but these never saw market adoption, probably because you'd only see a modest increase in potency at best, while requiring a harder to obtain precursor.
Interestingly, benzos containing a trifluoroethyl group on the 1-nitrogen atom did see pharmaceutical use. One of these is halazepam (N-fluoroethyl-nordazepam), which was quickly withdrawn from the US market again because it didn't sell, however. The other and much more interesting one is quazepam. Quazepam and its metabolite 2-oxoquazepam apparently exhibit much higher selectivity for the sleep-inducing subunits of the GABA receptor than other benzos, similar to the z-drugs zolpidem and zaleplon.
This analog of caffeine is said to be 100 times more active at both the A1& A2 receptors.
During WWII the Germans needed coffee to keep their war industry going 24 hours/day. Due to the Allied embargo on coffee beans, ersatz coffee was made using synthetic caffeine. I've seen the synthesis method in an Allied intelligence report & it is not simple, beginning with relatively complex raw materials like ethyl cyanoacetate. A significant amount of German war productivity went into making synthetic coffee, as did the production of their popular soft drink, FANTA. In the case of FANTA, coloring & flavoring came from industrial byproducts because importation of the original drink syrup from Coca-Cola was also blocked..

During WWII the Germans needed coffee to keep their war industry going 24 hours/day. Due to the Allied embargo on coffee beans, ersatz coffee was made using synthetic caffeine. I've seen the synthesis method in an Allied intelligence report & it is not simple, beginning with relatively complex raw materials like ethyl cyanoacetate. A significant amount of German war productivity went into making synthetic coffee, as did the production of their popular soft drink, FANTA. In the case of FANTA, coloring & flavoring came from industrial byproducts because importation of the original drink syrup from Coca-Cola was also blocked..

Last edited:
Organic azides do not tend to be the most stable compounds, to the point where inorganic azide salts and low-molecular weight organic azides tend to be shock-sensitive explosives.... not to mention azide salts as well as several organic azides being fairly toxic.
Trifluoromethyl groups shouldn't pose any such problem. I know of trifluoromethyl analogues of nordazepam (triflunordazepam) and clobazam (triflubazam), but these never saw market adoption, probably because you'd only see a modest increase in potency at best, while requiring a harder to obtain precursor.
Interestingly, benzos containing a trifluoroethyl group on the 1-nitrogen atom did see pharmaceutical use. One of these is halazepam (N-fluoroethyl-nordazepam), which was quickly withdrawn from the US market again because it didn't sell, however. The other and much more interesting one is quazepam. Quazepam and its metabolite 2-oxoquazepam apparently exhibit much higher selectivity for the sleep-inducing subunits of the GABA receptor than other benzos, similar to the z-drugs zolpidem and zaleplon.
I agree with your comments on quazepam (DORAL) regards inducing sleep.
You got the linear nomenclature for halazepam wrong. See attached graphic. Intermediates derived from 2,2,2-trifluoroethanol, are commonly available on the market. The correct nomenclature is N-(2,2,2-trifluoroethyl)-.

Last edited:
sekio
Bluelight Crew
Anyone had any first-hand experience with [atomoxetine]?
I once took 80mg and it made me feel like I'd been hit by a truck while simultaneously having the flu. In retrospect it's probably a massive overdose but I was young and dumb...
I once took 80mg and it made me feel like I'd been hit by a truck while simultaneously having the flu. In retrospect it's probably a massive overdose but I was young and dumb...
40 mg is the STARTING dose for ADHD. Assuming you don't have ADHD, it may have acted like someone dropping a case of beer on your brain.
Be cautious when contemplating even small lab-scale ozonlysis. I worked in a lab where we made tens of kilos of a perfume ingredient using an industrial-scale ozonator (>$19K) & had to run the ozonolysis in ice-cold methylene chloride in 22-liter flasks. I don't think Walmart sells either CH2Cl2 or elemicin.
Because of both ambient heat & the heat of reaction, keeping those big flasks at around 0 deg C was a lot of work. The product passed & I got paid
Because of both ambient heat & the heat of reaction, keeping those big flasks at around 0 deg C was a lot of work. The product passed & I got paid
Last edited:
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
JacksinPA,
I already have elemicin. But as watched as I am [think permanent Saturday Night Live actor status], I am not *about* to do anything funny with it.
And I did 100mg 5-MAPB once. Hard to describe altered state of consciousness that ended in mild delirium--I desperately called my dad around 11 pm although he was just one room away. Will not voluntarily repeat.
AMP
I already have elemicin. But as watched as I am [think permanent Saturday Night Live actor status], I am not *about* to do anything funny with it.
And I did 100mg 5-MAPB once. Hard to describe altered state of consciousness that ended in mild delirium--I desperately called my dad around 11 pm although he was just one room away. Will not voluntarily repeat.
AMP
Non-benzodiazepine full Bz receptor agonist(s)
I only have access to the first page of the full journal article but this caught my eye:

For some reason I could not recover the full article through Sci-Pub.
Interesting from the SAR POV. Wonder what other analogs they produced & evaluated. Not lab-scale chemistry in any event.
I only have access to the first page of the full journal article but this caught my eye:

For some reason I could not recover the full article through Sci-Pub.
Interesting from the SAR POV. Wonder what other analogs they produced & evaluated. Not lab-scale chemistry in any event.
Last edited:
According to the book QD 1 A355 no. 45 p. 117,
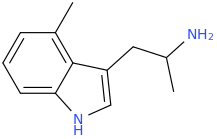
MP-809
Is "particularly effective in the treatment of neurotic depressions" and "is only a weak MAOI."
4-Methylindole-3-carboxaldehyde is a commercially available product.
- Status
- Not open for further replies.








