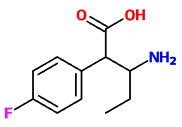
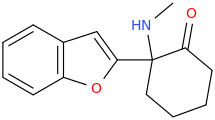
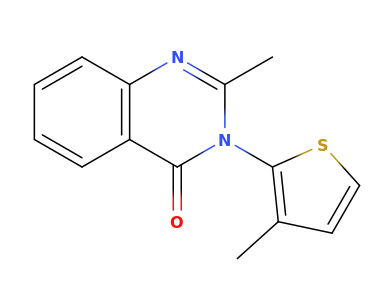
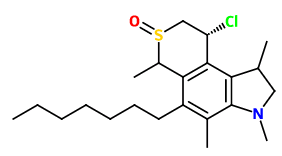
Lorcaserin is apparently a shit experience so I wouldn't really care to pursue it.. 
I am not sure what you mean by 'similar patterns', but for starters you would have to know exactly how they bind - in what spatial orientation. I have found differences in binding orientation between what appear to be very analogous drugs to be very surprising and that would totally ruin SAR assumptions and hybrids like you are talking about. But, if you do know that they bind in a certain analogous orientation I think hybrids can be fair ideas since the function of groups and their positions have an okay chance of matching.
We're talking hybrids of compounds with at least partially matching activity here. Making hybrids of drug molecules that aren't really in the same category very rarely works because the modifications aren't tolerated by either 'side' which gives you something in the middle which is accepted by none of the receptors. bk-2C-B is one of the rare exceptions and it takes advantage of the fact that within the realm of PEAs there is already an overlap of psychedelics and stimulants. It is another thing to try and find a completely new overlap!
Silicium no never heard of that. @ the MDMA idea: while silicium can bind strongly to oxygen, the methylenedioxy bridge there on the left is a really tricky thing. It is a sort of acetal which are normally unstable but here it is stabilized by conjugation and resonance with the adjacent aromatic ring. I think it has been shown to tolerate very little change like messing with the methylene function. What I am saying is: the silicium doesn't necessarily have to go exactly there in the molecule if you want to make a replacement... it could be a bad idea or there is the off chance that it turns out particularly great. Still: not sure if there is a reason to take such chances.
N-benzyl functions like NBOMe or NBOH can make for pretty selective 5-HT2A agonists when PEAs or very closely related compounds are fit with them, but that seems to be a sort of exception. Even fitting psychedelic tryptamines with them doesn't seem to work and many of those are 5-HT2A agonists too.
I think that means it doesn't bode well for trying to use it for other serotonin receptor subtypes since even for 2A it is so particular. Maybe though there is a way to make adjustments to try and find an analogy for tryptamines that *does* work. It would require investigating how they bind different spatially to the receptor so that you can try to model it. Possibly you can use a similar group meant to bind to the same part of the 2A site, but it may need to be bound differently to tryptamines than would be casually guesstimated, or it may take an extra spacer.
I am not sure what you mean by 'similar patterns', but for starters you would have to know exactly how they bind - in what spatial orientation. I have found differences in binding orientation between what appear to be very analogous drugs to be very surprising and that would totally ruin SAR assumptions and hybrids like you are talking about. But, if you do know that they bind in a certain analogous orientation I think hybrids can be fair ideas since the function of groups and their positions have an okay chance of matching.
We're talking hybrids of compounds with at least partially matching activity here. Making hybrids of drug molecules that aren't really in the same category very rarely works because the modifications aren't tolerated by either 'side' which gives you something in the middle which is accepted by none of the receptors. bk-2C-B is one of the rare exceptions and it takes advantage of the fact that within the realm of PEAs there is already an overlap of psychedelics and stimulants. It is another thing to try and find a completely new overlap!
Silicium no never heard of that. @ the MDMA idea: while silicium can bind strongly to oxygen, the methylenedioxy bridge there on the left is a really tricky thing. It is a sort of acetal which are normally unstable but here it is stabilized by conjugation and resonance with the adjacent aromatic ring. I think it has been shown to tolerate very little change like messing with the methylene function. What I am saying is: the silicium doesn't necessarily have to go exactly there in the molecule if you want to make a replacement... it could be a bad idea or there is the off chance that it turns out particularly great. Still: not sure if there is a reason to take such chances.
N-benzyl functions like NBOMe or NBOH can make for pretty selective 5-HT2A agonists when PEAs or very closely related compounds are fit with them, but that seems to be a sort of exception. Even fitting psychedelic tryptamines with them doesn't seem to work and many of those are 5-HT2A agonists too.
I think that means it doesn't bode well for trying to use it for other serotonin receptor subtypes since even for 2A it is so particular. Maybe though there is a way to make adjustments to try and find an analogy for tryptamines that *does* work. It would require investigating how they bind different spatially to the receptor so that you can try to model it. Possibly you can use a similar group meant to bind to the same part of the 2A site, but it may need to be bound differently to tryptamines than would be casually guesstimated, or it may take an extra spacer.