Frigyeslayman
Greenlighter
- Joined
- Jul 3, 2017
- Messages
- 1
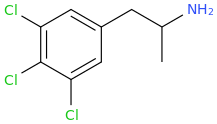
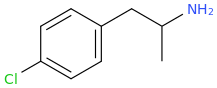
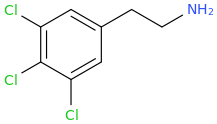
What does this make? It sounds like a pcp synthesis
5.3g of Bleach (sodium hypochlorite) (chlorine) and acetic acid (lemon juice) and salt None Iodized this is cyclohexane Synthesis
sodium carbonate is added with rapid stirring distilled
3.93g of potassium cyanide
8.17ml of piperine is added after it is created into piperdine (black pepper works) then cooled on ice heated in microwave
Magnesium turnings(irosply alcohol and wash containing magnesium sulfate) were added dried 30ml of (ether,gasoline,alcohol) and then bromozine-arisol (any type of fragrance)
Then toluene is added (or acetone) and sharpie ink /(calcium chloride)
add 10.3ml of anhydrous (baking soda) add
several grams of ammonium chloride and sodium hydroxide is added (drain cleaner) ice again then dilute 3 times with hydrocloric acid cool in the freezer until it's dry then unfreeze into a liquid
5.3g of Bleach (sodium hypochlorite) (chlorine) and acetic acid (lemon juice) and salt None Iodized this is cyclohexane Synthesis
sodium carbonate is added with rapid stirring distilled
3.93g of potassium cyanide
8.17ml of piperine is added after it is created into piperdine (black pepper works) then cooled on ice heated in microwave
Magnesium turnings(irosply alcohol and wash containing magnesium sulfate) were added dried 30ml of (ether,gasoline,alcohol) and then bromozine-arisol (any type of fragrance)
Then toluene is added (or acetone) and sharpie ink /(calcium chloride)
add 10.3ml of anhydrous (baking soda) add
several grams of ammonium chloride and sodium hydroxide is added (drain cleaner) ice again then dilute 3 times with hydrocloric acid cool in the freezer until it's dry then unfreeze into a liquid