MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,746
I call this one Bromo-Tripioid. https://ibb.co/NTmdBp2
N&PD Moderators: Skorpio | someguyontheinternet
This one reminds me of β-Phenylmethamphetamine.I call this one Super-MDMA. https://ibb.co/379MDfC
Are you saying
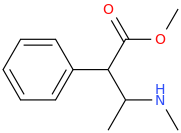
BOUNCE
1-phenyl-1-carbomethoxy-2-methylaminopropane
doesn't work?
Tryptamine and ergoline analogues are not not really my thing.
Maybe next time I get something speedy, I'll try to post your molecules, but some of them seem super hard to name with IUPAC to the point that I'm not sure what you mean.
Try giving the names to me one at a time?
Also, many of them are not correctly named. For example, you can't just say "phenyl etc. etc. 2,3-furan etc. etc. amine" because the 2,3-furan won't come up right in the drawing.
Are you saying

BOUNCE
1-phenyl-1-carbomethoxy-2-methylaminopropane
doesn't work?
Tryptamine and ergoline analogues are not not really my thing.
Maybe next time I get something speedy, I'll try to post your molecules, but some of them seem super hard to name with IUPAC to the point that I'm not sure what you mean.
Try giving the names to me one at a time?
Also, many of them are not correctly named. For example, you can't just say "phenyl etc. etc. 2,3-furan etc. etc. amine" because the 2,3-furan won't come up right in the drawing.
not a drug , please but a usefull precursor, put extra actyl on the 4-hydroxy.to inhibit dimers .What do you think of this as a drug?

4-HT (4-HydroxyTryptamine)
1-(4-hydroxyindole-3-yl)-2-aminoethane
the Frog returns.
How come?I regret to inform you that I will not be posting anymore Drug Designs.
The tetrazole group is easy you just react the intermediate nitrile with sodium azide.
There are many examples of this in the literature ranging from phenyltropanes to sartan antihypertensives.
Nufenoxole is an interesting synthesis. The azole is reacted with acetic anhydride for the last step.
I know a long list of compounds with 4 consecutive nitrogens. If you've read Danny's books, i'm sure you know that Molinazone is the one that is mentioned there.
