Deinonychus
Bluelighter
- Joined
- Oct 20, 2012
- Messages
- 401
Tried to clean up this thread, some posts were very borderline and at times I edited out the rotten and off-topic parts. I really don't care if you think the moderating was fair, if you fail to make posts that are less than 10% of a contributing value it is your own responsibility and it may be effectively hidden/removed or edited.
If a discussion stalls it really doesn't help to reflect on that uselessly.
Hmm. Dunno if this is for me or in general, i'd hope in general since I'd hope I've contributed more than ten percent value, but as I'd just made critical comments about moderation on Bluelight, I'm not sure. Perhaps I should explain better what I meant, and surely I don't actually dispute that cleaning out the thread is needed, if thats the impression you got.
My comment was directed to it'sALLfake, because he was less than thrilled that Sekio – being in theory 'authoritative' – made a fact-ish sounding statement that dirty acid is a figment of human imprecision and variability. My point was simply that mods here do that sort of statement as fact thing a lot, amongst a few other disagreements I have with what seems to be the purposefully coordinated tone of the moderation on the whole site. It is a touch too authoritarian and occasionally harsh and often too impersonal for my tastes you see.
Perhaps this is necessary with so large a site, and surely there will inevitably be difficulties because moderators are members of the forum too and doubtless wish to engage in debate and discussion without inviting chaos for a visit by visibly relinquishing any power in order to converse on a level playing field. Those things are complicating factors to me to be sure, but ultimately I am of the opinion that the sometimes totalitarian flavors are not necessary to such a degree. But in this scheme of things, my point was that the post it'sALLfake took issue with was really quite tame as far as throwing one's weight around as an authority who is also participating in a discussion.
I brought up fairness in moderating, but I wasn't talking about getting my posts pruned, if this comment was in actuality directed at me, as I'm not sure if it was. Instead I was talking in general terms, but this is a perfect example of what I was talking about: doing what you just did – pruning – is in my eyes the ideal for how a moderator works, whereas shutting down a thread like was threatened instead due to ease of doing so is not the ideal.
To compare with what I mean about authoritarian tendencies, this is a properly modulated response, whereas shutting the whole damn thread down would be overly authoritarian for my tastes. You hadn't actually pruned this thread yet when I made that post, so I was speaking in more general terms. Then again you could be talking to somebody else entirely as mentioned.
As far as pruning vs locking a thread goes in practice rather than in theory, I totally get that moderators are above all else volunteers. So real life time constraints are in play in a major way. But all the same I should hope that instead of doing the easy, fast thing, a moderator would wait until they did have the time, and then do the right thing instead of the fast and easy thing, right being in my opinion keeping a thread alive if it has value. Two people that can't get along shouldn't deprive everybody else of a venue for discussion just because that's the quick fix in other words.
Anyway it doesn't really matter since it did get pruned instead of locked, so thanks for taking the time to do it right instead of doing it quick.
Fortunately the fundamental idea behind the thread is a perennial topic and it seems valuable to continue working on piecing together that FAQ. I will try to help with that.
For me the subject is not 100% finished even if I swung over to the side that thinks that subjectively experiencing the quality of LSD is for the most part an illusion. I am not willing to put to rest the possibility that impurities that are inactive by themselves may be of influence on the complex pharmacology of LSD itself. The term 'modulation' was used for this.
It would have been great if there was more comment on that but I guess it is not surprising since no decently scientific minded person would claim that it is 100% impossible, and there is no empirical data to help us. So many stay silent and prefer to echo for the millionth time a conclusion that others have established using their own reasoning.
What would be necessary is a double blind large scale study where LSD together with different synthesis impurities (each separately purified on their own) are administered. Which is pretty unrealistic to happen, especially if the only application is the main Q in our FAQ.
Even if the great variance in physical effects from LSD accounts for somewhere between 90 and 100 % of the subjective experience of dirty or clean effects, it remains IMO possible that the remaining influence comes from impurities.
It is a form of wishful thinking or a form of inability to let go of wanting a more tangible cause, but at the same time I would personally feel scientifically incorrect to not even consider that option possible given what we know and don't know.
If LSD has such a great range (or variance) it is still possible IMO that this range is skewed by different batches. But if there are many factors the purity definitely doesn't seem like a deciding factor to me.
Right-o, this is on the mark. Since I haven't gotten feedback on any of the FAQ bits (no, I'm not whining, just stating the facts. FAQts, actually) would it trouble you to look over the most recent one, the one that gets at the question of what properties a contaminant would need? I'm really not wanting my bias on the subject to spoil my contribution to the FAQ, so I tried to be explicit that there could be an as of yet undiscovered ergoloid that is responsible, or a sub-active dose of a contaminant might be enough to color the experience, etc. but I'm just not sure that I included enough caveats to the text so as to be clear that there is still a possibility, however unlikely, of it being a real phenomenon, since we lack definitive scientific data on the subject.
On the other hand I don't want to go too far with the caveats. But considering that I'm biased against dirty acid rather than for it, that's the form of bias I would be most concerned about, so I would like to make sure that I made the chance of the phenomenon being real explicitly enough. I also am writing up a piece on how ergoloids work their side-effecty magic, so as to speculate how an ergoloid contaminant could cause the dirty acid syndrome side effect set, while also being clear that it is pure speculation, and that LSD is an ergoloid itself, so it would be very difficult to determine whether acid itself or some unknown ergoloid would be responsible for the syndrome.
Anyway if you're too busy that's cool, would just prefer somebody with an eye for the unconfirmed nature of the argument in either direction take a look and make sure my bias isn't showing!
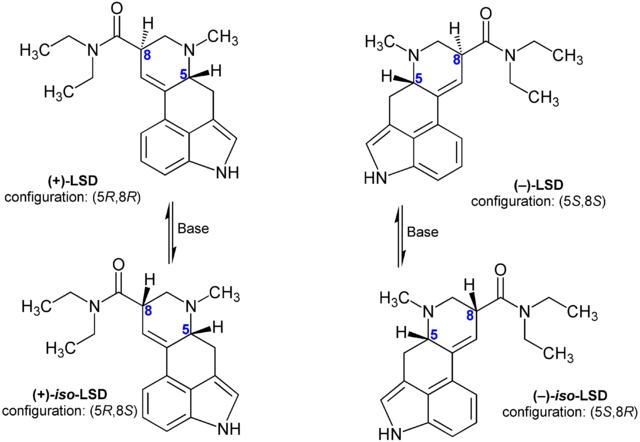
Since LSD and iso-LSD are diastereomers, not enantiomers, the equilibrium concentration ratio does not have to be 1.
Hmm. As far as I know enantiomers by definition cannot interconvert at all; if they did they wouldn't be enantiomers, since enantiomers require a symmetry reversal. So in the case of acid, (+)-LSD and (–)-LSD are enantiomers, and (+)-iso-LSD and (–)-iso-LSD are enantiomers, and the (+) form of each cannot interconvert at all to the (–) form.
I am not sure how (+)-LSD and (+)-iso-LSD interconvert though. These two are *not* enantiomers, just as you stated. However they do have a difference in one of there stereogenic centers, the 8-carbon stereocenter, where the hydrogen or the carbon of the amide can apparently switch orientations. I would hypothesize however than since the ring where the 8-carbon is located is *not* aromatic, the substituents that project from that 8-carbon in that ring could then rotate freely, or at least can actually rotate even if there's a low energy barrier to it. A 90 degree rotation would bring the amide's carbon and the hydrogen into opposite orientations than they had previously held, and since the carbon that hosts the amide ketone can also freely rotate, the 90 degree rotation around the point that is the 8-carbon wouldn't affect the amine's orientation much since it would be rotating around all the time anyway.
You're also correct that the interconversion of the (+) forms of LSD and iso-LSD respectively need not have an equilibrium of 1:1. If there was one orientation that was more energetically favorable, either because that orientation is inherently more favorable as a ground state, or because the energy barrier to rotation was more favorable in one direction that the other even if the resulting states themselves were equally favorable energetically, we would indeed see a value for the equilibrium that diverged from 1. But in that event we would eventually see almost the acid turn to either d-LSD or iso-LSD, and since this interconversion doesn't require light (in contrast to the irreversible change to lumi-LSD does require light) then you'd expect that equilibrium shift to happen even in the proverbial 'time-capsule' LSD, that being the Sandoz product somebody apparently stashed away for half a century in the dark and cold.
The cold would slow down interconversion as it slows any chemical reaction, but there should still be noticeable change in the potency due to an equilibrium constant in either direction that deviates from 1:1 for a half-century old bottle. But when it was tried out, it was apparently just the same as the day it was made. This leads me to believe that the interconversion from (+)-LSD to (+)-iso-LSD and back again has a ratio of 1:1 (and the same reaction between (–)-LSD and (–)-iso-LSD would also have a similar ratio, though there shouldn't be any (–) in play due to synthetic precursors lacking that pair of enantiomers, and wouldn't matter anyway since both are inactive). This is basically inference since acid that is stored properly doesn't turn to either almost all d-LSD or almost all iso-LSD even over 50 years, in summary.
Curiously but sensibly (digressing but still on the topic of chirality and interconversion), the totally chemistry-beginner (I could myself amongst this group) would consider that nitrogen, perhaps as an amine, would be capable of producing a chiral center, since it too has tetrahedral form like carbon. And the answer lies in that one of the 'points' of the tetrahedron is occupied by nitrogen's lone pair electrons. Since these electrons aren't being shared in a bond with anything, they 'orbit' the nucleus freely... except that remember, electrons don't *really* orbit a nucleus like planets, this is just an abstraction, and in reality they occupy *orbitals*, which are essentially probabilistic functions of where the electrons are likely to be found at any given time. So as a result they can 'jump' around the nucleus (there is no jumping, they are just varyingly likely to be found at a given place in one part of the orbital as the other, and relative locations of the three actual bonds the nitrogen has do not stop this probabilistic 'movement') allowing the other three bonds to change their absolute orientations to one another. This is why enantiomers amines do not exist: they interconvert with themselves constantly, so even an isolated sample of a given enantiomer if such a thing were to simply pop into being as a pure thing, it would rapidly (not sure how fast but is assume super fucking almost instantly fast) become racemic due to the lone pair electrons wandering around the nucleus.
EDIT: Also, thanks for the list Sekio, I guess I've got a bunch more reading to do. I knew how broad the binding affinity for acid was – serotonin, dopamine, adrenaline receptors of many subtypes – but I guess for some reason I thought it was atypical in this regard. I guess not! Thinking back on that reasoning now I can't figure out why is make such a weird, perverse assumption. Oh well, tis to be human, and I'm off to google around on receptor subtypes then!
Last edited: