phase_dancer
Bluelight Crew
From user reports, Dimethocaine HCl appears to be available in Australia. It could be used to cut cocaine, or mixed with other drugs/cuts etc , or sold straight. It has been referred to as 'legal cocaine' and seems to have acquired the street name larrikin (no doubt after the name generic name larocaine).
Background
As the unwanted side effects of cocaine were apparent early on, synthetic anaesthetics were developed shortly after its structure had been determined. Eucaine was first synthesised in 1859, but proved too toxic. Elucidation of the essential part of the cocaine molecule led to piperocaine, which was non-habit forming and less toxic than cocaine. Procaine was eventually developed and used for many years, again because of its non-habit forming properties and low toxicity (~1/4 that of cocaine). Like several of the synthetic anaesthetic esters, Procaine contains an para-amino substituted aromatic ring. Other para amino esters include Benzocaine with a 2 carbon tail, and tetracaine with an n-butyl group.
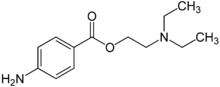 Procaine
Procaine
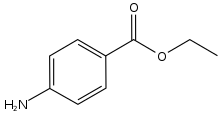 Benzocaine
Benzocaine
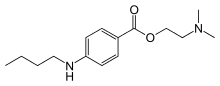 Tetracaine
Tetracaine
Dimethocaine was developed as a anaesthetic, although it's not commonly used for this purpose, if at all. It wouldn't have been a popular candidate for anaesthesia as it pales next to some other esters and has relatively high CNS activity.
Pharmacology
At first glance it might appear dimethocaine has little in common with cocaine apart from the benzoate group i.e. the carbonyl group attached to the aromatic ring.
 Dimethocaine
Dimethocaine
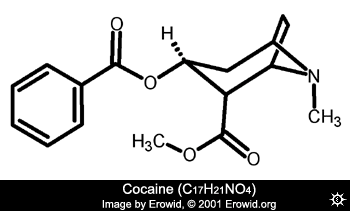 Cocaine
Cocaine
As said, the benzoate ester is a common feature of many of the synthetic anaesthetics. The side chain of the ester could be a simple carbon chain as in benzocaine, a linear chain tertiary amine as with procaine, or a branched chain as with dimethocaine. If a tertiary amine is present, one of the condition necessary for CNS activity is the correct proximity of the amine to the carbonyl oxygen and the carboxyl oxygen. The branched chain of dimethocaine threfore places the amine in a spatially similar position to the azabicyclo nitrogen of cocaine.

As can be seen, the 3D or spatial model of cocaine superimposes quite well over that of dimethocaine (
 for the image Hammilton) indicating it could act like cocaine in terms of its CNS activity; binding to DAT and preventing DA uptake.
for the image Hammilton) indicating it could act like cocaine in terms of its CNS activity; binding to DAT and preventing DA uptake.
Dimethocaine is reportedly ~ 1/3 to 1/2 the potency of cocaine, and produces a similar euphoria, although some people claim a noticeable difference. From the user reports on BL, most people agree the effects last 1- 1/2 hours, then abruptly cease.
http://www.bluelight.ru/vb/search.php?searchid=4086599
Metabolism
Cocaine and the synthetic ester ‘caines’ are chiefly metabolised via hydrolysis, and dimethocaine is expected to be handled in a similar manner, producing 4-amino benzoic acid (aka para-aminobenzoic acid or PABA) and 3-(diethylamino)-2,2-dimethylpropanol.
PABA is produced in humans from bacteria in the bowel. It was also once used extensively as a UV filter in sunscreen, however, it was found that PABA increases the formation of a DNA defect and increases the risk of skin cancer. In some animals (not humans) it is a biological precursor for folate. PABA toxicity (in-vivo uses) has been noted in humans but most cases resulted from consuming large amounts (>10g/day) as a supplement. Some health companies state PABA stimulates healthy bowel flora when taken orally but there’s little evidence to back this up. Anyway, for most people this metabolite shouldn’t prove too troublesome with reasonable use patterns.
There is little available on the actions and toxicity of the second primary metabolite -- 3-(diethylamino)-2,2-dimethylpropanol. It was researched as a possible anti-tumour agent, but was found to be ineffective (check pub-med for review summary). With what’s available on this compound it’s impossible to fully deduce what possible problems it could cause. It’s expected 3-(diethylamino)-2,2-dimethylpropanol will be oxidised to the carboxylic acid and readily excreted (Note; comments welcome here)
Genetic Predisposition
A particular health concern needs to be mentioned first, although is not likely to affect many users. Those suffering from a condition known as Pseudocholinesterase Deficiency must avoid the use of many drugs, particularly esters that are metabolised by this enzyme. For PD sufferers, taking such drugs can result in severe, life threatening situations (see wiki entry for Pseudocholinesterase deficiency). While the disease is generally rare, above normal prevalence among some ethnic groups has been noted.
Toxicity
The other, perhaps more relevant health concern involves the basic actions of the drug - the anaesthetic and CNS effects. Coke causes many problems in this regard, with hospital admissions due to heart related problems common with long term/ heavy use. Those with underlying heart conditions are particularly at risk, possibly even from relatively low and infrequent dosages. From first appearances it was thought dimethocaine could have anaesthetic actions equal or greater than an equivalent dose of cocaine. Thankfully, it would appear it only has around half the anaesthetic potency of cocaine, but that doesn't mean it's safe. While internet prices for dimethocaine HCl vary greatly, it can currently be sourced from some chem suppliers at a fraction of the price of cocaine, which could well mean larger dosages are consumed (remember you need around twice that of cocaine for a similar effect). So, the warnings in relation to the dangers of cocaine also apply here. It may even turn out that dimethocaine is far more toxic to the heart, but there seems to have been little research done in this area so no-one really knows.
Cocaine is also known to cause many health problems, particularly with heavy use. It can be reasonably expected that many of these could also apply to dimethocaine use, perhaps even at lower dosages.
Identification
Cocaine can be subjected to various tests to evaluate it's purity. Scott's test (cobalt thiocyanate) can test for the presence of coke but it also reacts with some other compounds, and potassium thiocyanate is said to be able to evaluate the purity of cocaine. But by far the most common tests done are melting point and the Chlorox or bleach tests. In the past these have been considered a fairly reliable means of establishing purity, particularly when both tests are performed. However, dimethocaine has a melting point very close to that of cocaine (196-197°C and 195°C respectively). Reports indicate dimethocaine may also fool the bleach test.
In looking for a suitable reagent test for dimethocaine, the para-amino group is a good target, but unfortunately most tests involve the use of toxic or difficult to handle chemicals. One of the least dangerous of the reagent tests involves the use of Coniferyl alcohol. 0.3 g is heated until it melts, then dissolved in a few mls of ethanol, and diluted to 10ml. A drop of a solution of the sample is placed on filter paper, the coniferyl alcohol soln added, and the paper exposed to HCl vapours.
Legality
As mentioned, in terms of spatial orientation, dimethocaine is certainly a structural analogue of cocaine. However, in terms of the respective functional groups there is quite a difference. Cocaine is a di-ester, dimethocaine isn't. Cocaine has a tropane ring, whereas dimethocaine has a branched, alkylamine, and dimethocaine has an aromatic amine, where coke doesn't.
To my limited understanding of legal things, I would imagine this could prove to be a technical hurdle for prosecutors, and may require testament on the pharmacological similarities in order to convict. Has a precedent ever occurred in Australia where substantially similar pharmacological actions have been successfully used? If not, it may well have to be if prosecutions result from dimethocaine use. And this stuff is undoubtedly only the beginning. There are countless possibilities here, some of which will again be considerably different in terms of the functional group/placement and structure.
It would be good to hear the thoughts of some of our BLers more familiar with analogue legislation; fortehlulz, Tabaluga, Biscuit.... and from anyone with reports of dimethocaine use, particularly in regards to any observed side effects, interactions with other drugs etc.
Background
As the unwanted side effects of cocaine were apparent early on, synthetic anaesthetics were developed shortly after its structure had been determined. Eucaine was first synthesised in 1859, but proved too toxic. Elucidation of the essential part of the cocaine molecule led to piperocaine, which was non-habit forming and less toxic than cocaine. Procaine was eventually developed and used for many years, again because of its non-habit forming properties and low toxicity (~1/4 that of cocaine). Like several of the synthetic anaesthetic esters, Procaine contains an para-amino substituted aromatic ring. Other para amino esters include Benzocaine with a 2 carbon tail, and tetracaine with an n-butyl group.



Dimethocaine was developed as a anaesthetic, although it's not commonly used for this purpose, if at all. It wouldn't have been a popular candidate for anaesthesia as it pales next to some other esters and has relatively high CNS activity.
Pharmacology
At first glance it might appear dimethocaine has little in common with cocaine apart from the benzoate group i.e. the carbonyl group attached to the aromatic ring.


As said, the benzoate ester is a common feature of many of the synthetic anaesthetics. The side chain of the ester could be a simple carbon chain as in benzocaine, a linear chain tertiary amine as with procaine, or a branched chain as with dimethocaine. If a tertiary amine is present, one of the condition necessary for CNS activity is the correct proximity of the amine to the carbonyl oxygen and the carboxyl oxygen. The branched chain of dimethocaine threfore places the amine in a spatially similar position to the azabicyclo nitrogen of cocaine.

As can be seen, the 3D or spatial model of cocaine superimposes quite well over that of dimethocaine (

Dimethocaine is reportedly ~ 1/3 to 1/2 the potency of cocaine, and produces a similar euphoria, although some people claim a noticeable difference. From the user reports on BL, most people agree the effects last 1- 1/2 hours, then abruptly cease.
http://www.bluelight.ru/vb/search.php?searchid=4086599
Metabolism
Cocaine and the synthetic ester ‘caines’ are chiefly metabolised via hydrolysis, and dimethocaine is expected to be handled in a similar manner, producing 4-amino benzoic acid (aka para-aminobenzoic acid or PABA) and 3-(diethylamino)-2,2-dimethylpropanol.
PABA is produced in humans from bacteria in the bowel. It was also once used extensively as a UV filter in sunscreen, however, it was found that PABA increases the formation of a DNA defect and increases the risk of skin cancer. In some animals (not humans) it is a biological precursor for folate. PABA toxicity (in-vivo uses) has been noted in humans but most cases resulted from consuming large amounts (>10g/day) as a supplement. Some health companies state PABA stimulates healthy bowel flora when taken orally but there’s little evidence to back this up. Anyway, for most people this metabolite shouldn’t prove too troublesome with reasonable use patterns.
There is little available on the actions and toxicity of the second primary metabolite -- 3-(diethylamino)-2,2-dimethylpropanol. It was researched as a possible anti-tumour agent, but was found to be ineffective (check pub-med for review summary). With what’s available on this compound it’s impossible to fully deduce what possible problems it could cause. It’s expected 3-(diethylamino)-2,2-dimethylpropanol will be oxidised to the carboxylic acid and readily excreted (Note; comments welcome here)
Genetic Predisposition
A particular health concern needs to be mentioned first, although is not likely to affect many users. Those suffering from a condition known as Pseudocholinesterase Deficiency must avoid the use of many drugs, particularly esters that are metabolised by this enzyme. For PD sufferers, taking such drugs can result in severe, life threatening situations (see wiki entry for Pseudocholinesterase deficiency). While the disease is generally rare, above normal prevalence among some ethnic groups has been noted.
Toxicity
The other, perhaps more relevant health concern involves the basic actions of the drug - the anaesthetic and CNS effects. Coke causes many problems in this regard, with hospital admissions due to heart related problems common with long term/ heavy use. Those with underlying heart conditions are particularly at risk, possibly even from relatively low and infrequent dosages. From first appearances it was thought dimethocaine could have anaesthetic actions equal or greater than an equivalent dose of cocaine. Thankfully, it would appear it only has around half the anaesthetic potency of cocaine, but that doesn't mean it's safe. While internet prices for dimethocaine HCl vary greatly, it can currently be sourced from some chem suppliers at a fraction of the price of cocaine, which could well mean larger dosages are consumed (remember you need around twice that of cocaine for a similar effect). So, the warnings in relation to the dangers of cocaine also apply here. It may even turn out that dimethocaine is far more toxic to the heart, but there seems to have been little research done in this area so no-one really knows.
Cocaine is also known to cause many health problems, particularly with heavy use. It can be reasonably expected that many of these could also apply to dimethocaine use, perhaps even at lower dosages.
Identification
Cocaine can be subjected to various tests to evaluate it's purity. Scott's test (cobalt thiocyanate) can test for the presence of coke but it also reacts with some other compounds, and potassium thiocyanate is said to be able to evaluate the purity of cocaine. But by far the most common tests done are melting point and the Chlorox or bleach tests. In the past these have been considered a fairly reliable means of establishing purity, particularly when both tests are performed. However, dimethocaine has a melting point very close to that of cocaine (196-197°C and 195°C respectively). Reports indicate dimethocaine may also fool the bleach test.
In looking for a suitable reagent test for dimethocaine, the para-amino group is a good target, but unfortunately most tests involve the use of toxic or difficult to handle chemicals. One of the least dangerous of the reagent tests involves the use of Coniferyl alcohol. 0.3 g is heated until it melts, then dissolved in a few mls of ethanol, and diluted to 10ml. A drop of a solution of the sample is placed on filter paper, the coniferyl alcohol soln added, and the paper exposed to HCl vapours.
Legality
As mentioned, in terms of spatial orientation, dimethocaine is certainly a structural analogue of cocaine. However, in terms of the respective functional groups there is quite a difference. Cocaine is a di-ester, dimethocaine isn't. Cocaine has a tropane ring, whereas dimethocaine has a branched, alkylamine, and dimethocaine has an aromatic amine, where coke doesn't.
To my limited understanding of legal things, I would imagine this could prove to be a technical hurdle for prosecutors, and may require testament on the pharmacological similarities in order to convict. Has a precedent ever occurred in Australia where substantially similar pharmacological actions have been successfully used? If not, it may well have to be if prosecutions result from dimethocaine use. And this stuff is undoubtedly only the beginning. There are countless possibilities here, some of which will again be considerably different in terms of the functional group/placement and structure.
It would be good to hear the thoughts of some of our BLers more familiar with analogue legislation; fortehlulz, Tabaluga, Biscuit.... and from anyone with reports of dimethocaine use, particularly in regards to any observed side effects, interactions with other drugs etc.
Last edited:




