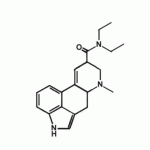
Throwing a meaty bone in here on f&b's suggestion of the beta-methylene substituted psychedelics as a mimick of the LSD double bond.Methinks he's onto something,might give the phenethylamines the "G spot" stimulator on the 5-HT2A receptor.Surely such compounds can be made.Though it looks we might get some MAO activity,but that hasn't been a negative.Below are the closest structures known,I fear the structures won't come through:
Stabase-protected 2-chloroallylamine: a useful synthon for primary allylic amines via nickel-catalyzed cross-coupling. Bargar, Thomas M.; McCowan, Jefferson R.; McCarthy, James R.; Wagner, Eugene R. Indianapolis Cent., Merrell Dow Res. Inst., Indianapolis, IN, USA. Journal of Organic Chemistry (1987), 52(4), 678-81. CODEN: JOCEAH ISSN: 0022-3263. Journal written in English. CAN 106:102001 AN 1987:102001 CAPLUS
Abstract
Grignard reagents couple effectively with the stabase protected form I of 2-chloroallylamine in the presence of 0.005-0.01 mol equiv. of [Ph2P(CH2)3PPh2]NiCl2 to provide an improved synthesis of 2-substituted allylamines. For example, 2-thienylmagnesium bromide was converted to thiopheneethanamine II in a one flask procedure in 78% yield. II is useful as an inhibitor of dopamine b-hydroxylase.
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Unsaturated heterocyclic amines as potent time-dependent inhibitors of dopamine b -hydroxylase. Bargar, Thomas M.; Broersma, Robert J.; Creemer, Lawrence C.; McCarthy, James R.; Hornsperger, Jean Marie; Palfreyman, Michael G.; Wagner, Joseph; Jung, Michel J. Indianapolis Cent., Merrell Dow Res. Inst., Indianapolis, IN, USA. Journal of Medicinal Chemistry (1986), 29(3), 315-17. CODEN: JMCMAR ISSN: 0022-2623. Journal written in English. CAN 104:129726 AN 1986:129726 CAPLUS
Abstract
RC

CH2)CHR1NHR2 (I, R = 2-thienyl, 3-thienyl, 2-furyl, 3-furyl, Ph; R1, R2 = H, Me) were prepd. and tested for dopamine b-hydroxylase inhibition. Thus, RC

CH2)Me (R = 2-thienyl) was treated with N-chlorosuccinimide to give RC

CH2)CH2Cl, which was treated with K phthalimide and then N2H2, followed by HCl to give I.HCl (R = 2-thienyl, R1 = R2 = H) (II.HCl). II and also PhC.tplbond.CCH2NH2 were potent, time-dependent inhibitors of dopamine b-hydroxylase, having activities 1000-fold greater than that of I (R = Ph, R1 = R2 = H). PhC.tplbond.CCH2NH2 and I (R ¹ Ph, R1, R2 = H, Me) also showed antihypertensive activity in rats.
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Enzyme-activated irreversible inhibitors of monoamine oxidase: phenylallylamine structure-activity relationships. McDonald, Ian A.; Lacoste, Jean Michel; Bey, Philippe; Palfreyman, Michael G.; Zreika, Monique. Strasbourg Cent., Merrell Dow Res. Inst., Strasbourg, Fr. Journal of Medicinal Chemistry (1985), 28(2), 186-93. CODEN: JMCMAR ISSN: 0022-2623. Journal written in English. CAN 102:58260 AN 1985:58260 CAPLUS
Abstract
The title compds. I (R and R1 = H, Br, Cl or F; R2 = H or OMe; R3 = H, OH, OMe or CF3; R4 = H, Cl, OH, Me, or OMe) were prepd. as HCl salts by several routes and evaluated in vitro by incubation with a crude rat brain mitochondrial monoamine oxidase (MAO) prepn., and in vivo in mice. With the exception of (Z)-3-chloro-2-phenylallylamine, which acted as a competitive inhibitor, all I caused a time-dependent inhibition of MAO when selective substrates were used. (E)-3-Fluoro-2-(4-methoxyphenyl)- and 2-(3,4-dimethoxyphenyl)-3-fluoroallylamine were as selective for the B form of MAO as deprenyl. (E)-3-Fluoro-2-phenylallylamine given to mice at 1 mg/kg, i.p., gave good inhibition of MAO in the brain and heart which lasted £48 h. Structure-activity relations were discussed.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Characterization of a Series of 3-Amino-2-phenylpropene Derivatives as Novel Bovine Chromaffin Vesicular Monoamine Transporter Inhibitors. Perera, Rohan P.; Wimalasena, D. Shyamali; Wimalasena, Kandatege. Department of Chemistry, Wichita State University, Wichita, KS, USA. Journal of Medicinal Chemistry (2003), 46(13), 2599-2605. Publisher: American Chemical Society, CODEN: JMCMAR ISSN: 0022-2623. Journal written in English. CAN 139:100849 AN 2003:347315 CAPLUS
Abstract
A series of 3-amino-2-phenylpropene (APP) derivs., e.g. I (R1 = H, F, Cl, HO, Me, MeO, etc.; R2 = H, HO, MeO; R3 = R4 = H, Et; R3 = Me, R4 = H), was synthesized and characterized as novel competitive inhibitors, with Ki values in the mM range, for the bovine chromaffin granule membrane monoamine transporter(s) (bVMAT). Although these inhibitors are structurally similar to the bVMAT substrate tyramine, none of them were measurably transported into the granule. Structure-activity studies have revealed that, while the 3- or 4-OH groups on the arom. ring enhance the inhibition potency, Me or OMe groups in these positions reduce the inhibition potency. Halogen substitution on the 4-position of the arom. ring causes gradual increase of the inhibition potency parallel to the electron donor ability of the halogen. Substituents on the NH2 as well as on the 3-position of the alkyl chain reduce the inhibition potency. Comparative structure-activity analyses of APP derivs. with tyramine and the neurotoxin 1-methyl-4-phenylpyridinium suggest that the flexibility of the side chain and the relative orientation of the NH2 group may be crit. for the efficient transport of the substrate through the bVMAT. Comparable bVMAT affinities of these inhibitors to that of DA and other pharmacol. active amines suggest that they are suitable for the structure-activity and mechanistic studies of monoamine transporters and may also be useful in modeling the mechanism of action of amphetamine-related derivs.
-------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Abstract
RR1C:CR2CH2NHR3 (R, R1 = H, F, Cl, Br; R2 = Ph, substituted Ph, naphthyl, indenyl, fluorenyl, heterocyclic; R3 = H, alkyl, CH2P, CH2CH2Ph) were prepd. Thus, treating R4CH2CO2CMe3 [R4 = 3,4-(MeO)2C6H4] ClCO2Et in ClCHF2 gave EtO2CCR4(CHF2)CO2CMe3, which was decarboxylated, dehydrofluorinated, and reduced to give (E)-HOCH2CR4:CHF (I). I was aminated with phthalimide and hydrazinolized to give (E)-II. (E)-II×HCl showed a selectivity for MAO-B inhibition of 500 relative to MAO-A inhibition.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------