fastandbulbous
Bluelight Crew
Back to LSD
Just going back to that comment you made before
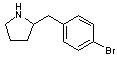
The modified LSD molecule shown below should form a hydrogen bond in the same way that the 5-hydroxy group of serotonin (or 2-methoxy of DOM)does. It shouldn't have any steric hinderance problems, as that part of the receptor can easily accomodate a furan ring. A 5-methoxy group would be preferable to a hydroxy group, as the latter's polarity would cause a slower penetration of the blood brain barrier.

I'm unaware of any natural compound that contains a lysergic acid group with a methoxy/hydroxy group in the position shown. It would require a total synthesis, starting from a 5-methoxyindole compound. As such, I dont think that we're likely to see it appear anytime soon
Just going back to that comment you made before
I'm confused, You've got the hydrogen bond pocket that the 5-methoxy group would fit into (the top one)... but what fits into that in LSD
The modified LSD molecule shown below should form a hydrogen bond in the same way that the 5-hydroxy group of serotonin (or 2-methoxy of DOM)does. It shouldn't have any steric hinderance problems, as that part of the receptor can easily accomodate a furan ring. A 5-methoxy group would be preferable to a hydroxy group, as the latter's polarity would cause a slower penetration of the blood brain barrier.
I'm unaware of any natural compound that contains a lysergic acid group with a methoxy/hydroxy group in the position shown. It would require a total synthesis, starting from a 5-methoxyindole compound. As such, I dont think that we're likely to see it appear anytime soon