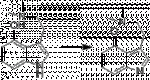"4-MeO-DET is completely without action either orally or by smoking at dosages up to 30mgs."
"4-MeO-MIPT:
"(with 26mg, orally) This is my first try with this drug, ever. First indication of effects in twenty minutes. Quiet onset, no remarkable visuals, in fact no visuals at all. To a +2 within about ten or fifteen minutes more. Body is comfortable, mind-set pretty much unchanged from baseline. No euphoria, no insights. But also, no discomfort. Erotic was extremely successful, and orgasm seemed easier than with other materials. Still no visuals but seemed to be a soft +3. Music fine. Hard to define exactly how we knew we were in an altered state, because of the lack of visual cues. Body aware more than mind. Would like to explore this further. Perhaps for writing? Nice material. Maybe higher next time?"
Conclusion:
4-MeO-DMT is worth trying, but I doubt that it's active. Maybe try 4-(CCl3)-DMT instead.
On second thought, 4-(CF3)-DMT is more like it. Those three chlorine atoms are probably too bulky.