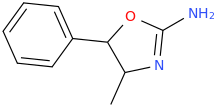
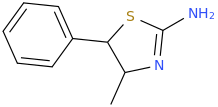
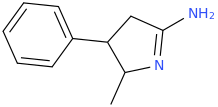
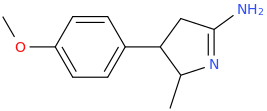
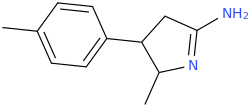
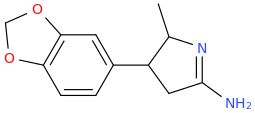
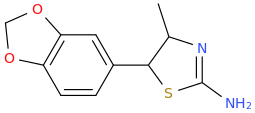
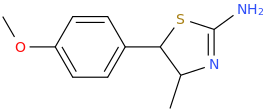
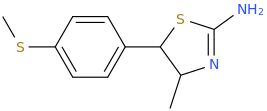
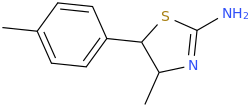
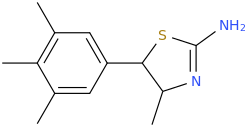
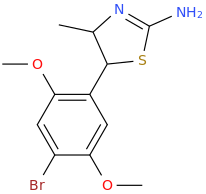
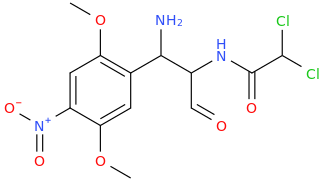
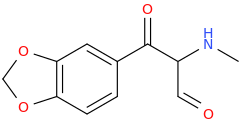
Generation *DMT*
Greenlighter
- Joined
- Aug 23, 2023
- Messages
- 10
Who won the world series this year 2023?
N&PD Moderators: Skorpio | thegreenhand
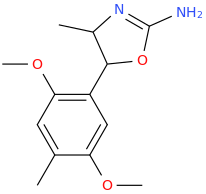
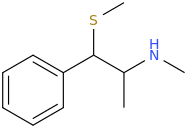
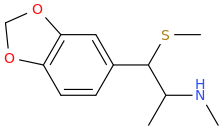
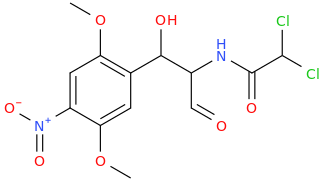
Is it a kappa agonist?My limited knowledge of diprenorphine is that it's restricted use is due to it's side-effects, specifically hallucinations and dysphoria.
^ not a new compound at all. However the potency in vivo is nowhere near as high as is suggested by in vitro. ED50 32ug/kg in guinea pigs. In humans it is not that potent (fentanyl level potency)
Patent is 1972 to Sanko company, Chem Pharm Bull (Japan) 2050-2057 (1970)
I don't understannd this obsession with in vitro potency over everything, are you playing some kind of smackhead top trumps?
Safer opioids would be a good thing and super potent /= safer
Dezocine based compounds with reduced respiratory depression and or ceiling effect would be a better approach if harm reduction really was your priority
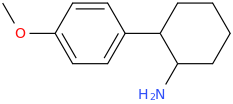
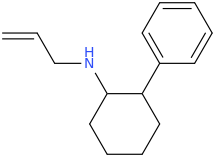
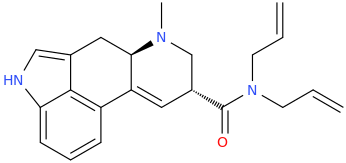
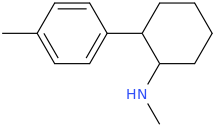
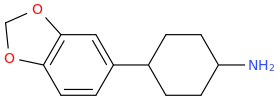
Is it a kappa agonist?