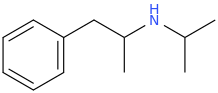
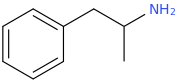
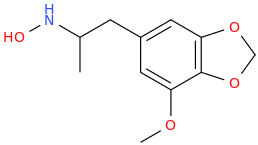
AlsoTapered
Bluelighter
(1R,5S)-9-methoxy-9-phenyl-3-(2-phenylethyl)-3-azabicyclo[3.3.1]nonane
(1R,5S)-9-methoxy-9-phenyl-3-(2-phenylethyl)-3-azabicyclo[3.3.1]nonane | C23H29NO | CID 21151698 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
BTW the above compound is an example of an opioid demonstrated to be over x100,000 morphine in potency.
If one has access to ChemOffice, it's worth overlaying the above compound with fentanyl, BDPC and similar compounds. The above compound hasn't seen optimization such as replacing the beta-aromatic with a 2-thienyl or 2-furanyl moiety or the addition of a chiral beta-hydroxy moiety.
But in a PM the Canadian BLer noted that researchers used OHMEfentanyl as a reference compound and were shocked to discover that even after 3 applications of OHMEfentanyl, the compound was not dislodged from the MOR receptors.
When we reach these levels of potency, Ki values are not measured in micromoles (uM) but rather in nanomoles (nM). I have mistakenly read affinity data ASSUMING the values were in uM only to discover upon closer examination that the values were in nMs.
The paper 'A MOR Antagonist with High Potency and Antagonist Efficacy among Diastereomeric C9-Alkyl-Substituted N-Phenethyl-5-(3-hydroxy)phenylmorphans'
Although the above paper ostensibly discusses antagonist, it's important to note that opiate ligands which display antagonist activity MUST include a meta-hydroxy moiety on the alpha aromatic (benzene) ring or bioisostere thereof.