fastandbulbous
Bluelight Crew
I wouldn't feel so confident over swapping a sulphur atom for an oxygen. 2,4,5-trimethoxyphenethyl amine is ineffective unless a MAOI is administered at the same time. On the other hand, replacing the 4-oxygen atom with sulphur gives 2C-T, which works on its own orally. Equally aleph-1 (DOT) is a much more potent inhibitor of MAO than TMA-2.Thinking about the metabolic fate of that ring made me very worried. However, it appears that 2-aminothiazolines occur in the human body:
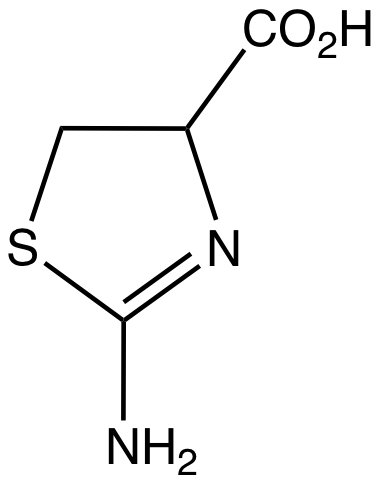
2-Aminothiazoline-4-carboxylic acid - Wikipedia
en.wikipedia.org
So this may not be so dangerous at least in that way.
Anyway, aminorex is reported to be a monoamine oxidase inhibitor! Unfortunately replacing O by S usually doesn't affect this activity; cf. PMA and 4-MTA. So I'm skeptical of the safety anyway.
On the other hand N-ethylaminorex might be worth looking into. Ethyl isocyanate is a pretty nasty reagent, though!
My theory is that the replacement of the 4 oxygen with a sulphur, allows the compound to occupy the active site, due to ssimila electronic structure, but the sulphur prevents the enzyme from taking the active conformation, hence a competitive MAO. Think, on it's own, when snorted (rapid onset), proved fatal. As the alkyl group on the sulphur gets bigger, the more potent an MAOI it is.