flying-potato
Greenlighter
- Joined
- Sep 4, 2015
- Messages
- 39
4-metoxymethyl-methcathinone :


N&PD Moderators: Skorpio | thegreenhand

The two molecules I drew are mostly planar.
But they aren't in real life unless you make them rigid and even then, the lowest energy-state might not be planer.
Fluoroadamantate is an interesting idea.
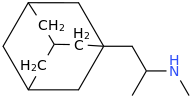
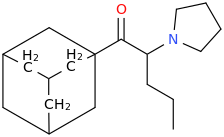
Requires an aromatic (flat & binding in lipophilic pocket via Van der Waals forces.
Would an endo-etheno bridge on a cyclohexane fit to that specification or is it not flat by its internal linkage (as rendering tools usually default it to a boat conformation if its not held in a specific way to other constitutents)?
Since the double bonds rotate in benzene, is there another conformation where the same would be the case, holding a stable rotating type structure within a 2D-planar fixed molecule?
It would make the structure even "more three-dimensional", besides no conformation of cyclohexane is really flat with half of the hydrogen atoms being axial while in aromatics hydrogen atoms are perfectly aligned with the plane of the ring. Aromatic rings usually interact with aromatic rings of amino acid residues (in some cases they can also interact with carboxyl groups, which are also flat, proper substitution with an electron-withdrawing group on the ring is necessary, I guess, as plain alkylbenzene moiety is not electronically predisposed for such an interaction, e.g. cocaine's ester interacting with a tyrosine residue at DAT). It's certainly the case for stimulants related to amphetamine which when bound to DAT has its aromatic ring placed in a region with a few aromatic rings to interact with. Look up propylhexedrine which has mainly adrenergic effects. Wikipedia states it has psychostimulant properties at doses much higher than therapeutic ones, but somehow I doubt it could get any better than levo-methamphetamine at any dose.
What do you mean by that? The bonds constituting the ring can't rotate for sure, they're not really double bonds either, each carbon atom of the benzene ring has an unhybridized p orbital with one electron in it and these 6 p orbitals create a pi system in which all 6 bonds are equivalent in length.
BTW, a few weeks ago we discussed whether 1-(2,3-dihydro-1,3-benzoxazol-6-yl)propan-2-amine would be a stable compound, I've checked and it might be. Apparently benzoxazoline can be easily made in a similar manner as benzodioxole, no sophisticated methods needed. N-acylbenzoxazolines seem stable with respect to the position between N & O atoms, so plain benzoxazoline should be stable enough to survive subsequent steps in the synthesis towards the target compound.
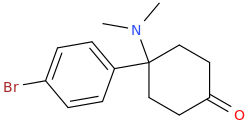
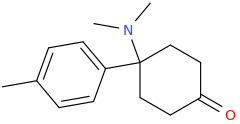
Thanks for putting that up, N. I had to do U47700 and it was sheer hell. They KNEW it was important but were concerned that only PRIMARY sources were referenced. I mean - THAT IS ALL THAT'S OUT THERE!
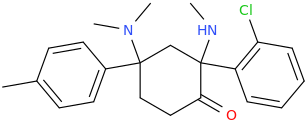
I would strongly recommend that you also add Lednicer's parent compounds for BDPC. 4-(dimethylamino)-4-(4-methylphenyl)cyclohexan-1-one & 4-(4-bromophenyl)-4-(dimethylamino)cyclohexan-1-one were both equipotent. I asked him why he hadn't tried MDPC... and he said that he simply forgot! WHY a bromine & methyl worked equally well for the simple cyclohexanones is a mystery - but an interesting one.
I really think you should obtain a copy of Chemoffice by hook or by crook. I want us all to be able to just use SMILES to transfer compounds and the IUPAC name is generated. I don't know how much the student edition costs but it's like Word for the PC. Yes, there alternatives but even in The Ukraine, they use it, allowing me to describe compounds to them. I know it isn't cheap, but when you have it, you will wonder how the hell you managed before.
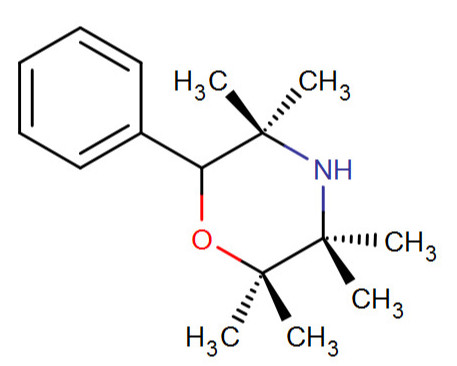
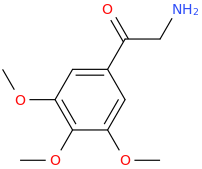
RC fodder.
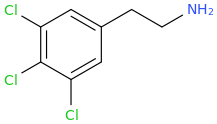
2C-SHIVA, a known stimulant but not, reportedly, a psychotomimetic.
anyone ever heard of an n-feruloylserotonin?
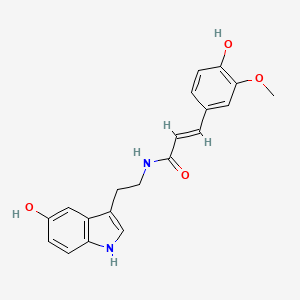
I wonder if you could stick a number of other tryptamines where the serotonin is? the study on n-feruloylserotonin said it had selective effects on stress in rodents, who knows what it does to humans but its in a number of preworkout supplements, including mine probably (considering theres carthamoides extract in mine)
I'd love to know if anyone finds any intriguing info on n-feruloylserotonin or related compounds
