In addition to 5-HT, melatonin, DA, NE, and glutamate etc., I think this would make an awesome addition to our current family of neurotransmitters. No idea how to go about that one, though, unless it is through some kind of extensive natural selection following a designer gene insertion:
3,4,5-trihydroxyphenylethanamine.
This is the stuff of aliens, I believe.
Sound far fetched? Well, sure, but then again, some kind of octopus (and many insects and, to a lesser extent, humans as a TAAR agonist) uses octopamine:
Octopamine.
(Then again, scientists who recently did some DNA study on octopuses finally reached the conclusion that they may actually be an alien life form.)
I also think it's a shame we don't produce this neurotransmitter:
N-methyl-dopamine.
N-Me-DA is the reason MDMA is so rewarding, and if you can figure that relationship out on your own, then you're smarter than most, I would say.
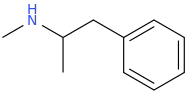
But, like I said last week or the week before maybe, this molecule is really all we need:
2-methylamino-1-phenylpropane.
It's a good place to start, anyway. Notice how it has no oxygen molecules anywhere in its constitution to lower its potential energy (oxidized organic compounds are basically partly like ash, which of course always has a lower potential energy than whatever was burned ["rapidly oxidized"] to produce that ash). Also, see how its basic form is supremely fat soluble and ready to cross into the brain at a moments notice, while its protonated N form is perfectly water soluble if I understand that right and ready to be absorbed by the acidic aqueous solution found in our stomachs. Finally, note how sturdy the molecule is; so sturdy, in fact, that 50% of a dose in humans is excreted unchanged in our urine. Yes, I'm on it again.