neurotic
Bluelighter
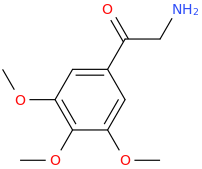
What about an alpha methyl homologue of that??? Seems promising enough to me. The a-methyl doesn't get in the way of psychedelic activity... (Most?) DOx are even more potent than their 2-Cx counterparts, and that beta keto group doesn't seem to get in the way of 5-HT2a agonism either (thinking of BK-2C-B... It is not a very potent drug though...)

Looks sexy too...
Last edited: