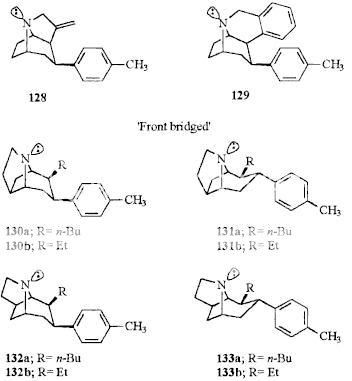
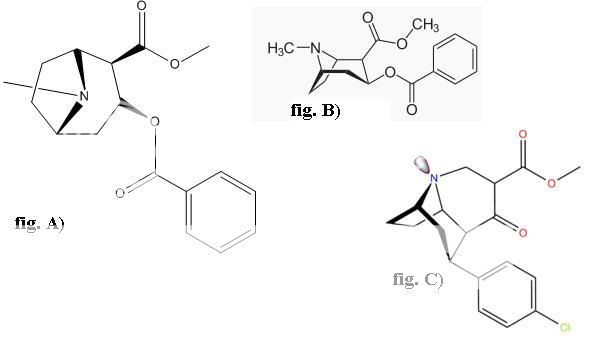
Wittig reaction can do that, with the phosphonium salt made from MOM-Cl and a trialkylphosphine (I don't know if PPh3 is the best, and they don't work so well on ketones sometimes, so maybe there are better conditions) and is undergrad level.
Wouldn't that be a nonstandard use of the Wittig reaction? The wikipedia entry shows the carbonyl being replaced with a C=C double bond, which looks vaguely familiar from o-chem all those years ago. Looks too complex for undergrad.