paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
this compound
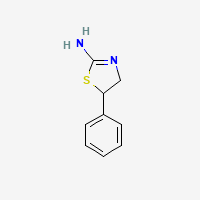
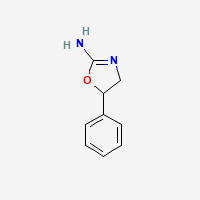
a thiazoline the sulfur homolog of aminorex, an oxazoline:
was discussed here on BL some time ago..apparently sold online by some stupid a..holes having no fckng idea WTF they doing. THIS IS NOT SAME AS AMINOREX or related stimulants like cyclazodone. we tried that one back in BC. It is highly potent triple releaser, somewhere between mdma and meth..more like meth actually but least 5x as potent as d-meth. It is EXTREMELY COMPULSIVE on par with d-methamphetamine! or crack.. Be careful ! There is nothing in the literature about that compound psychoactivity profile afaik.. but correct me if I am wrong. the closest would be thiadrine (priatan), drug developed in germany for asthma in 60s
incidentally some of the thiadrine side-effects including psychiatric intoxication and sympatomimetic typical of stimulants startin at 25 mg PO(will post ref later..
do not assume because chemical structure is very similar to aminorex or even cyclazodone type stimulants, it would have same pharmacology profile!!!. so please be extremely careful if yo u happen come across it! Stay Safe Out There
a thiazoline the sulfur homolog of aminorex, an oxazoline:
was discussed here on BL some time ago..apparently sold online by some stupid a..holes having no fckng idea WTF they doing. THIS IS NOT SAME AS AMINOREX or related stimulants like cyclazodone. we tried that one back in BC. It is highly potent triple releaser, somewhere between mdma and meth..more like meth actually but least 5x as potent as d-meth. It is EXTREMELY COMPULSIVE on par with d-methamphetamine! or crack.. Be careful ! There is nothing in the literature about that compound psychoactivity profile afaik.. but correct me if I am wrong. the closest would be thiadrine (priatan), drug developed in germany for asthma in 60s
incidentally some of the thiadrine side-effects including psychiatric intoxication and sympatomimetic typical of stimulants startin at 25 mg PO(will post ref later..
do not assume because chemical structure is very similar to aminorex or even cyclazodone type stimulants, it would have same pharmacology profile!!!. so please be extremely careful if yo u happen come across it! Stay Safe Out There
