-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
MXE...meet OXA.
- Thread starter blueberries
- Start date
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
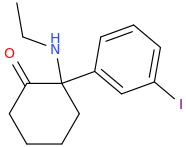
Tiletamine is impressive. Super-K if you like. I would swap the N-ethyl for an N-methyl just to reduce it's duration but since tiletamine is made commercially, the intermediates will be cheap.... and it makes sense to try the CHEAP options first.
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Reports of tiletamine itself are somewhat mixed. It's interesting to note that the QSAR of this class is sufficiently understood to make predictions. It is not clear if ring-substitution of the thiophene ring is worthwhile. It could be argued that the S of the thiophene ring should be counted as a substitution. While Reaxys listed a paper in which a furan ring was the aromatic, the paper could not be found.
I think it's time to move from the cyclohexyl moiety. It's presence is biosteric and the 4-thianes have been investigated and shown to be MORE active. That is, affinity increases. It would seem obvious to make the 4-thiane homologue of MXE and then 2-chloro MXE & then their N-methyl homologues. Quite a few compounds.
Of course, it's the fact that the (S) enantiomers are NMDA antagonists & the (R) enantiomers are DRIs that is crucial to subjective effects. It's very simply to produce pure DRI & pure NMDA antagonists by resolving the isomers or reading the QSAR of the classes to suppress one of the actions.
I think it's time to move from the cyclohexyl moiety. It's presence is biosteric and the 4-thianes have been investigated and shown to be MORE active. That is, affinity increases. It would seem obvious to make the 4-thiane homologue of MXE and then 2-chloro MXE & then their N-methyl homologues. Quite a few compounds.
Of course, it's the fact that the (S) enantiomers are NMDA antagonists & the (R) enantiomers are DRIs that is crucial to subjective effects. It's very simply to produce pure DRI & pure NMDA antagonists by resolving the isomers or reading the QSAR of the classes to suppress one of the actions.
Last edited:
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
It will be. MK-801 (dizocilpine) was developed to show that the 'magic angle' between aryl & N: is 107.5°. MK-801 also shows N-ethyl to be optimal. Kind of surprised people didn't just go for fluoroethyl or isopropyl or something similar. If the cuclohexyl moiety is banned via Murkush structural rules, thianes are MORE potent (in above link).
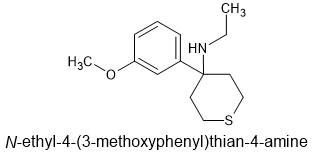
The above overlays MXE perfectly. Of course, it's metabolism would need some investigation. If the S is oxidised then the sulfone may display increased opioid activity (similar to dimetamine) BUT if it goes to the sulfonate, it's hard to predict. It's always a worry if you do not have a very clear picture of metabolism.
With the seeming lack of insight deployed in recent designs, it is inevitable that a toxic RC will reach the market. WHEN it does, the law will be fast to act.

The above overlays MXE perfectly. Of course, it's metabolism would need some investigation. If the S is oxidised then the sulfone may display increased opioid activity (similar to dimetamine) BUT if it goes to the sulfonate, it's hard to predict. It's always a worry if you do not have a very clear picture of metabolism.
With the seeming lack of insight deployed in recent designs, it is inevitable that a toxic RC will reach the market. WHEN it does, the law will be fast to act.
Last edited:
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
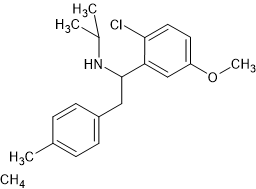
I think I have posted sufficient ref to show this is the obvioust homologue. Aryl iodides are not stable, if you want to alkylate your body, go ahead, use the m-I.
I would go for the N-methyl to see how it goes (it will have higher DRI than N-ethyl) AND if the N-methyl is banned, swap for the N-ethyl and if that is banned then go for the N-isopropyl.
That way you have 3 decent analogues.
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
BTW the time has come to discard the cyclohexylamine (although 4 thienyl can go back through all of the cyclohexyl analogus and the 1,2-diphenylethylamines. There are much more active compounds that cost less per dose to make.
Ring=substituted 8A-PDHQ still allows aromatic substitution. PD-137889 (with ring substitution & MK-801 (Diplizocine) with ring-substitution are the next step. OK, a lot harder to make, but 2-4mg is a dose. I guess 12,5mg of active with cut would be as potent as any of the ACA derivatives.
PD-137889 is the best and most convenient since ring-substitution allowa mu agonissm, DRI activity and controlled NMDA antagonism...... which is cool, presuming someone performs a QSAR.
I HATE the idea of 3mg active + 40mg cut. We need to find a ROA of the pure material.
Ring=substituted 8A-PDHQ still allows aromatic substitution. PD-137889 (with ring substitution & MK-801 (Diplizocine) with ring-substitution are the next step. OK, a lot harder to make, but 2-4mg is a dose. I guess 12,5mg of active with cut would be as potent as any of the ACA derivatives.
PD-137889 is the best and most convenient since ring-substitution allowa mu agonissm, DRI activity and controlled NMDA antagonism...... which is cool, presuming someone performs a QSAR.
I HATE the idea of 3mg active + 40mg cut. We need to find a ROA of the pure material.
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
It could be put on tabs. I'm not such a fan of the longer come-up time than nasal but perhaps geltabs would make it quicker (I'm not sure of the mechanics of jellies vs. cardboard but it seems like it should increase absorption time)?
Either that or just dropper vials with ethanol to make it slightly faster. 100mg/vial is roughly equal to 1g MXE dosages and the vials are cheap as chips on eBay.
Also I reckon the basic substances could be tweaked a bit for some alternatives ala PCP analogues but with far more analogues and variety.
PS: Sorry for bringing up an old thread but this last year has been a nightmare.
New year, new possibilities though!
Either that or just dropper vials with ethanol to make it slightly faster. 100mg/vial is roughly equal to 1g MXE dosages and the vials are cheap as chips on eBay.
Also I reckon the basic substances could be tweaked a bit for some alternatives ala PCP analogues but with far more analogues and variety.
PS: Sorry for bringing up an old thread but this last year has been a nightmare.
New year, new possibilities though!
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
It could be put on tabs. I'm not such a fan of the longer come-up time than nasal but perhaps geltabs would make it quicker (I'm not sure of the mechanics of jellies vs. cardboard but it seems like it should increase absorption time)?
Either that or just dropper vials with ethanol to make it slightly faster. 100mg/vial is roughly equal to 1g MXE dosages and the vials are cheap as chips on eBay.
Also I reckon the basic substances could be tweaked a bit for some alternatives ala PCP analogues but with far more analogues and variety.
PS: Sorry for bringing up an old thread but this last year has been a nightmare.
New year, new possibilities though!
Your original post IS MXE, not an analogue.
That is an image of 2-(3-Methoxyphenyl)-2-(ethylamino)cyclohexanone AKA MXE is it not?
So I'm not totally sold on your prowess as a drug designer.
The most active analogues have 4-thiane rings in place of the cyclohexane, the most potent ring-substitution appears to be 2-chloro-5-methoxy (based on the patents which detail some 400 analogues of ketamine. Of course, the Chinese might struggle to make the 4-thiane analogues but the 2-chloro-5-methoxy is certainly not covered... although fast&bulbous did note that the precursor is a bit more costly.
Littana
Bluelighter
- Joined
- Feb 4, 2012
- Messages
- 303
Your original post IS MXE, not an analogue.
^^ maybe click the link open and see more before getting all high up
- Joined
- Feb 8, 2006
- Messages
- 65,043
I found mxe to good but nowhere as good as ketamine which is the best ach I know of.
Personally, MXE is the only disso I have fallen in love with... it blows ketamine out of the water in every regard. Ketamine is okay... but just okay. Nothing to write home about. But we're all different, aren't we?
Reports of tiletamine itself are somewhat mixed. It's interesting to note that the QSAR of this class is sufficiently understood to make predictions. It is not clear if ring-substitution of the thiophene ring is worthwhile. It could be argued that the S of the thiophene ring should be counted as a substitution. While Reaxys listed a paper in which a furan ring was the aromatic, the paper could not be found.
I think it's time to move from the cyclohexyl moiety. It's presence is biosteric and the 4-thianes have been investigated and shown to be MORE active. That is, affinity increases. It would seem obvious to make the 4-thiane homologue of MXE and then 2-chloro MXE & then their N-methyl homologues. Quite a few compounds.
Of course, it's the fact that the (S) enantiomers are NMDA antagonists & the (R) enantiomers are DRIs that is crucial to subjective effects. It's very simply to produce pure DRI & pure NMDA antagonists by resolving the isomers or reading the QSAR of the classes to suppress one of the actions.
I believe the reason for the extremely mixed reviews of tiletamine are because all older reports I'm aware of are using veterinary preparations, which contain either strong benzos or other additional strong sedatives. Which muddys the waters substantially.
All of the more recent pure tiletamine reports I have read seem to be glowing.
Incidentally, I have in my possession 250mg of pure tiletamine (aka O-TCE) that I got recently... but I have yet to find the right time to try it. I will certainly report back.