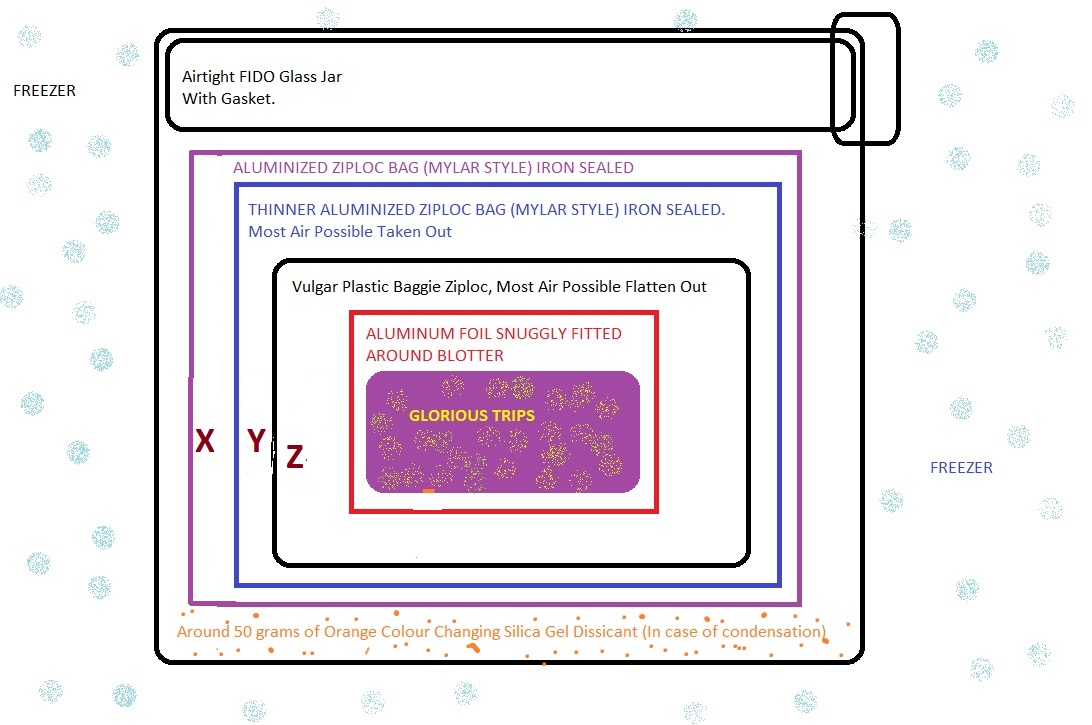
Every time I have stored LSD I have stored it in the freezer in foil. For shorter periods of time I have kept it in my wallet for a few hours. I've stored them for quite an extended period of time in the freezer and still experienced effects; however after awhile it will become useless in the freezer as well. I'd say after a 1-2 weeks it will start losing its impact, or at least this is what I typically experienced.
Seriously? LSD blotter should keep its potency for YEARS in the freezer