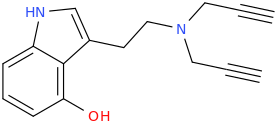
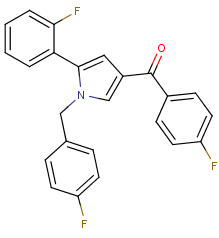
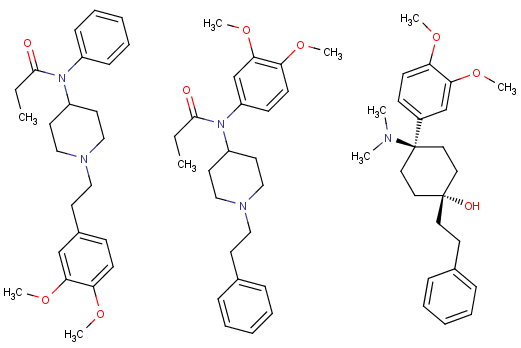
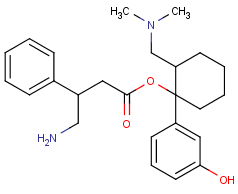
Cannabinoid with 3 fluoride punching fists
what happens if you put randomly dimethoxy´s at the phenyl units in drugs like fentanyl or bromadol? (It´s stupid but I like dimethoxy units, probably becouse of the sceletium tortuosum (kanna) alkaloids (mesembrine etc.))
Couldn´t a version of fentanyl in the potency of ocycodon be just as good? I thought those over-potent opioids don´t provide the (non tolerant) user as good experiences as lighter opioids so why don´t they just bring out the weakest analogues of fentanyl, bromadol etc. to the rc market?
edit: search for "marvin beans" (it´s free) the "MarvinSketch" sub-program is really fun to play with and you can learn many things about molecules. There´s also "Jchem" with a program that predicts metabolites etc. but I somehow can´t get it to work, weither online nor the desktop version (java errors at installation etc.).
would this metabolize into phenibut and o-desmethyl-tramadol?
edit: more phenibut things:
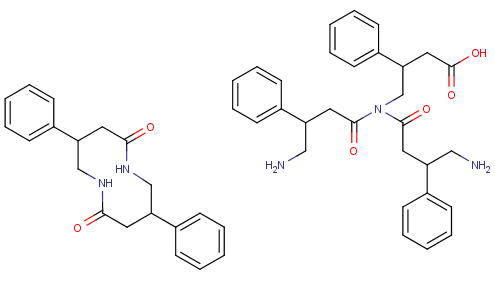
phenibut with an extra gaba chain
(I´m on clonitrazolam, always getting creative on benzos)
edit: what would happen if you would take for example if you had 100 similar compounds like different benzodiazepines, to make it easier, of similar strenght like 1mg Alprazolam, 1mg Etizolam, 1mg Flunitrazepam...etc. (or adjusted to equivalent dosages of compounds with different strenght) until you have a mix with 100mg; would this be effective at all? Like instead of a 1mg dose of one compund you get 100x10mcg of different but similar compunds, would they sum up to give a new effects or would the dose of each be too low? I had this question on mind for a long time, I wonder how it could change the pharmacology for different drugs.