-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
DMT from tryptophan - summary
Here is a general recap of my recent posts over months on converting store-bought L-tryptophan into DMT without the use of any hazardous or difficult-to-obtain reagents.
The classic approach to methylating that amine is to use methyl iodide. As a thought, methyl bromide is a commercial (and highly toxic) insecticide/fumigant & might be more readily available in cylinders from commercial sources that might not ask too many questions.
A gas at room temperature, methyl bromide readily penetrates skin, cloth, and other protective materials such as rubber and leather. It is nonflammable and toxic at low concentrations. Methyl bromide is odorless and odor provides no warning of hazardous concentrations.

Methyl iodide is extremely toxic, much like its relative methyl bromide. In one report a worker handling MeI suffered from symptoms severe enough that they were thought to be from a stroke. (See https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-4-177).
'A 41 YR OLD CHEMIST DEVELOPED METHYL IODIDE INTOXICATION. CHARACTERISTICS OF METHYL IODIDE POISONING INCLUDE DELAY BETWEEN EXPOSURE & ONSET OF SYMPTOMS, EARLY SYSTEMIC TOXICITY WITH CONGESTIVE CHANGES IN LUNGS & OLIGURIC RENAL FAILURE, PROMINENT CEREBELLAR & PARKINSONIAN NEUROLOGIC SYMPTOMS AS WELL AS SEIZURES & COMA IN SEVERE CASES, & PSYCHIATRIC DISTURBANCES THAT LAST FROM MONTHS TO YEARS.' (See https://monographs.iarc.fr/agents-classified-by-the-iarc/).
In/ a fatal case of poisoning in a worker exposed to methyl iodide fumes during its manufacture: severe neurological symptoms preceded death; all organs showed congestion at autopsy.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: http://monographs.iarc.fr/ENG/Classification/index.php p. V41 221 (1986)]
Even though the preps on Youtube look simple, MeI is nothing to fool around with.
Will this work?
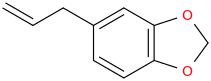
+ turpentine + O2 (g) -->

Oxidations of this sort usually involve DMF, CuCl2 & PdCl2. No turpentine.
This revised scheme seeks to get around the toxicity problems associated with MeI by using the simpler Finkelstein Reaction to generate benzyl iodide from benzyl chloride. My expectation is that reaction with the thiolate will selectively remove the benzyl group, leaving free DMT.

Benzyl methyl sulfide should not be too hard to handle as it is used as a food flavoring additive.

Benzyl methyl sulfide should not be too hard to handle as it is used as a food flavoring additive.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,993
Benzyl halides are lachrymators and are actually nastier to work with than MeI - MeI is toxic sure, but not stinky.
Also, any low m.w. methylsulfide would be stink-o-rama, thioanisole is particularly egregious "Has a solvent type odor and an solvent type flavor" - thanks good scents.... I'd bet that benzyl methyl sulfide would have ppb odor threshold.
Also, any low m.w. methylsulfide would be stink-o-rama, thioanisole is particularly egregious "Has a solvent type odor and an solvent type flavor" - thanks good scents.... I'd bet that benzyl methyl sulfide would have ppb odor threshold.
No, because of https://en.wikipedia.org/wiki/Pictet–Spengler_reaction will dominate the reaction, and i doubt any DMT will be found in the product.
A relatively recent paper on selective monp- & dimethylation of primary amines.
'Amines can be methylated when treated with formaldehyde and zinc in aqueous medium. Selective mono- or dimethylation can be achieved by proper choice of pH, stoichiometry and reaction time. This method can also be applied for amino acids.'
https://www.sciencedirect.com/science/article/pii/S004040390701711X

'Amines can be methylated when treated with formaldehyde and zinc in aqueous medium. Selective mono- or dimethylation can be achieved by proper choice of pH, stoichiometry and reaction time. This method can also be applied for amino acids.'
https://www.sciencedirect.com/science/article/pii/S004040390701711X

Bagseed
Bluelighter
- Joined
- Jul 22, 2010
- Messages
- 4,044
has anyone done that on tryptamine and reported on the yield? sounds to good to be true... no nasty alkyl halides involved.. formaldehyde and formic acid being easily available.Dresden, you've never heard of Eschweiler-Clarke methylation? Makes the aluminum amalgam reaction look like the utter mess that it is.
2eq. Formic acid, 2eq. formaldehyde, tryptamine, heat it. Done.
I still doubt on tryptamine on why it wouldnt yield carbolines as product, but I’ve done that many times on glutamic acid to make N,N-dimethylglutamic acid successfully. Fast and easy. (At times i was made it to make a CO2-strip cocatalyst in industrial product)
Only care to take is to effectively remove excess formaldehyde from crude.
Only care to take is to effectively remove excess formaldehyde from crude.
A relatively recent paper on selective monp- & dimethylation of primary amines.
'Amines can be methylated when treated with formaldehyde and zinc in aqueous medium. Selective mono- or dimethylation can be achieved by proper choice of pH, stoichiometry and reaction time. This method can also be applied for amino acids.'
https://www.sciencedirect.com/science/article/pii/S004040390701711X

Another reference (full paper): https://www.thevespiary.org/rhodium...ous-formaldehyde-and-zinc81ca.pdf?topic=262.0
Tryptamine + 2 eq. formaldehyde + HgCl2 + Heavy Duty Reynold's Wrap Al foil + MeOH --> DMT ?
Stay away from mercury salts or you may wind up 'Mad as a hatter.'
A big foreign chemical company I worked for developed this chemistry as a method of producing meta-trifluomethylphenyl-2-propanone, the raw material for one component of the subsequently banned diet drug fenfluramine (Fen-Phen). I happened on a copy of the synthesis, which was no longer of interest to them. As both the starting aniline & isopropenyl acetate are commercial products, this might be a possible path to making DOM (aka STP).
DOM was developed by Alexander Shulgin after he left Dow Chemical's former agrochemical research facility in Walnut Creek, CA. See https://patents.google.com/patent/US4105695?oq=ininventor:shulgin
As a side note, 2,5-dimethoxyaniline is also commercially available. Brominating it in the 4-position seems pretty straightforward to get to the potent DOM analog DOB. See https://en.wikipedia.org/wiki/2,5-Dimethoxy-4-bromoamphetamine. I've worked with 2,5-dimethoxyaniline & it is a very reactive molecule. It tends to react with oxygen to form dark orange-yellow nigraniline-type dyes, probably at the highly activated 4-position..


DOM was developed by Alexander Shulgin after he left Dow Chemical's former agrochemical research facility in Walnut Creek, CA. See https://patents.google.com/patent/US4105695?oq=ininventor:shulgin
As a side note, 2,5-dimethoxyaniline is also commercially available. Brominating it in the 4-position seems pretty straightforward to get to the potent DOM analog DOB. See https://en.wikipedia.org/wiki/2,5-Dimethoxy-4-bromoamphetamine. I've worked with 2,5-dimethoxyaniline & it is a very reactive molecule. It tends to react with oxygen to form dark orange-yellow nigraniline-type dyes, probably at the highly activated 4-position..


Last edited:
- Status
- Not open for further replies.

