Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
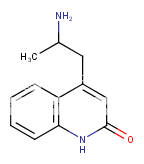
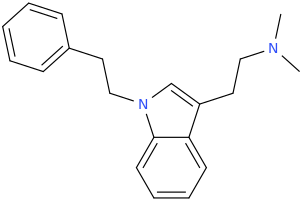
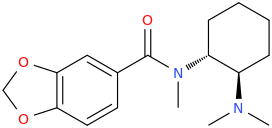
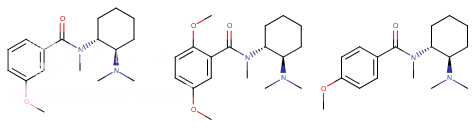
^ the 4-((2-dimethylamino)ethyl)quinolone work "well" as substitute for DMT: I made similar indoles isosteres back in the days!!Tryptophan analogs with the quinolone replacing the indole). It might work but lsd doesnt tolerate substitution at 2 position of the indole like with the 2-bromoLSD??) .. but who knows. NB: The one terrible thing about these types of quinolones, they're just terribly insoluble and I mean really pain in the ass to get them into any reasonable solvent.. unless you use boiling DMSO or DMF or .. cold conc sulfuric acid

If the problem is localized polarity, do you have any idea whether an emulsifier would work or a surfactant like HPBCD (HBC)?
Better to start off with 4-(dimethylamino)ethylquinoline anyway? Make that 1,2-dihydro-yadayadayada.
The effect of a 2-substitution of some tryptamines is known, primarily 2,N,N-TMT (Me-DMT) and I think also the 5-MeO analogue which I might actually have somewhere... they are active but quite different, like DiPT is "different" but apparently even differenter ;p.
It's definitely possible that 2-Me vs 2-Br is not comparable anyway, but I think regarding LSD many substitutions were tried and a lot on the indole nucleus were just not tolerated, don't know if 2-alkyls were among them but wouldn't be all that surprised if BOL made the cut.
LSD fits so snug that intolerance for substitutions doesn't necessarily translate that well to tryptamines or bioisosteres of trypts?
Last edited: