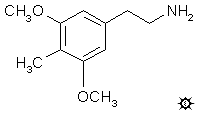
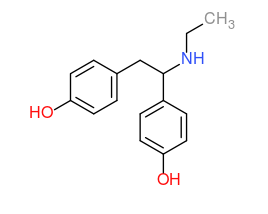
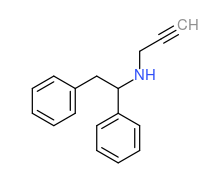
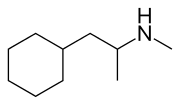
reminds me, the other night I had some interesting diarylethylamine ideas--
That's good idea (the top one): a dissociative opioid stim!
The (R) enantiomer may be a potent mu OP (~morphine, may be a bit less codeine?) (structure superimpose nicely on morphine!?)
and the (S) may be NMDA antagonist (superimpose on DXM) and Stim compare to (S)-PVP. Something along this line were discussed in earlier thread.(can't remember exactly):
morphinans with S stereoisomer at the C-N carbon=> NMDA antagonist
morphinans with R stereochemistry tend to give mu opioids
PS: very easy synthesis. What is the rationale for using propargyl (I mean in the second, the lefetamine analog
If anything the N-phenethylpiperidine is the more basic one, but by a small margin anyway. Also, N-piperidinyl a biostere of phenyl? In what way?
Thats why I qualify it:
Hopefully!..yes it is not really classical phenyl bioisostere like cyclohexyl (see METH analog
Propylhexedrine ). I figure, if the pKa of the N2 piperidinyl was low enough (< 6 ), the piperidine ring won't be charged at physiological so it might have a logP close to that of cyclohexyl. Therefore binds to the phenyl binding site with similar affinity to Ph. But of course, since it is the most basic it will be mostly protonotated at physiolocal pH so might not be the best replacement fo Ph. but if the difference of the pKa of the 2 Ns is not so large (eg 1 log unit) it might still work (10% will still be uncharged). Cyclohexyl may be better for this purpose.
The reason I thought about a piperidine there , it is way more easier to synthesize (straighforward synthesis by reacting piperidine with 1,2-dibromoethylbenzene! or substituted benzene)