crmt28
Bluelighter
- Joined
- Jun 19, 2014
- Messages
- 52
Orphenadrine is an anti-cholinergic with additional NMDA antagonism and norepinephrine reuptake inhibition. Looks pretty interesting. It's Diphenydramine with an additional methyl group.
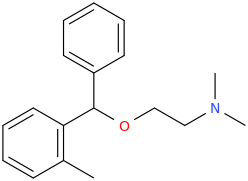
I wonder if removing the ethanolamine group could turn it into a pure NMDA antagonist. It would look pretty similar to Ephenidine.
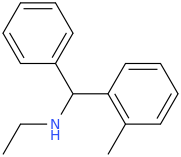
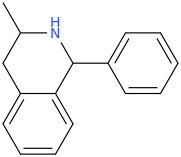
Close the ring and it turns into a possible stimulant?

I wonder if removing the ethanolamine group could turn it into a pure NMDA antagonist. It would look pretty similar to Ephenidine.

Close the ring and it turns into a possible stimulant?


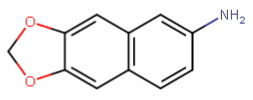
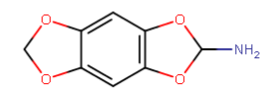
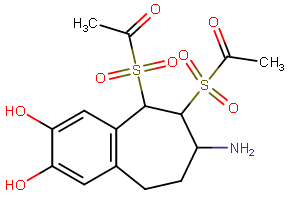
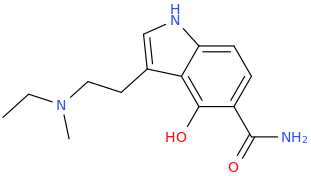
 there are several binding sites on the NMDAr with apparently in some ways a similar pharmacophore but with probably quite different tolerances for modified substitution on ligands for them (or maybe less than we think, when we discover nitrogen-free PCP site antagonists).. I guess that serves the role to have two (or more) very similar endogenous neurotransmitters being able to regulate different processes on a receptor complex.
there are several binding sites on the NMDAr with apparently in some ways a similar pharmacophore but with probably quite different tolerances for modified substitution on ligands for them (or maybe less than we think, when we discover nitrogen-free PCP site antagonists).. I guess that serves the role to have two (or more) very similar endogenous neurotransmitters being able to regulate different processes on a receptor complex.