chainsawkidd
Bluelighter
- Joined
- Nov 28, 2023
- Messages
- 27
First Bluelight post, greetings.
I will try to keep this relatively brief.
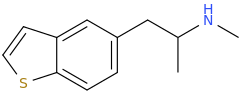
As I am very interested in drugs of the RC family, what subjects would one need to study in order to learn about how they are created? I understand that it would require years of dedication to reach the end goal, well aware, but we all start somewhere, could anyone please point me towards some helpful links/book PDFs/videos, anything related to the manufacturing of these compounds. Also, any helpful links for beginners of Chemistry, or whatever the exact subject related to my questions is.
Thankyou very much.
I will try to keep this relatively brief.
As I am very interested in drugs of the RC family, what subjects would one need to study in order to learn about how they are created? I understand that it would require years of dedication to reach the end goal, well aware, but we all start somewhere, could anyone please point me towards some helpful links/book PDFs/videos, anything related to the manufacturing of these compounds. Also, any helpful links for beginners of Chemistry, or whatever the exact subject related to my questions is.
Thankyou very much.