red22
Bluelighter
- Joined
- Nov 23, 2009
- Messages
- 1,224
Is there any significance in the fact that harmine, the MAOI traditionally used for DMT preparations,* is highly similar to serotonin? I have this idea that this fact conveys that harmine would be much more compatible with the brain than other MAOIs and inhibitor-type antidepressants, none of which are nearly as similar in structure to an endogenous chemical, as far as I know. I would imagine that the more dissimilar a chemical is from the brain's molecular foundations, the more it bangs things around when it enters the brain (in contrast to interacting smoothly with the brain); and inhibitor-type antidepressants including Nardil and Parnate,** two of the oldest MAOIs, definitely feel toxic in my experience.
For comparison purposes I'm listing other MAOIs below, including pictures of their structures, if it's useful. Maybe someone can say something about the comparison between their structures and harmine's structure.
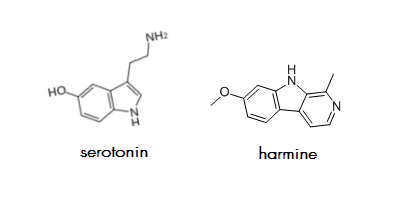
*Harmine is the predominant MAOI in Banisteropsis caapi vine.
Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson's disease. Samoylenko, V.; Rahman, M.; Tekwani, B. L.; Tripathi, L. M.; Wang, Y.; Khan, S. I.; Khan, I. A.; Miller, L. S.; Joshi, V. C.; Muhammad, I. Journal of ethnopharmacology. 10/2009; 127(2):357-67. DOI: 10.1016/j.jep.2009.10.030 DOWNLOAD
**Generic names: phenelzine and tranylcypromine.
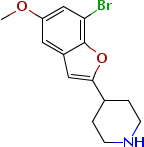
Chemical Name: brofaremine
Molecular Formula: C14H16BrNO2
C AS No.: 63638-91-5
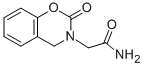
Chemical Name: caroxazone
Molecular Formula: C10H10N2O3
CAS No.: 18464-39-6
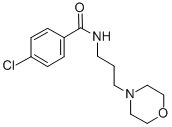
Chemical Name: eprobemide
Molecular Formula: C14H19ClN2O2
Formula Weight: 282.769
CAS No.: 87940-60-1
Chermical Name: amiflamina
Formula Weight: C12H20N2
CAS No.: 77697-37-1

Chemical Name: metralindole
Molecular Formula: C15H17N3O
CAS No.: 54188-38-4
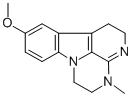
Chemical Name: minaprine
Molecular Formula: C17H24Cl2N4O
Formula Weight: 371.3
CAS No.: 25905-77-5
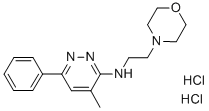
Chemical Name: pirlindole
Molecular Formula: C16H22N2O3S
Formula Weight: 322.42
CAS No.: 60762-57-4
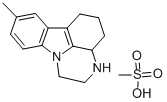
Chemical Name: toloxatone
Molecular Formula: C11H13NO3
Formula Weight: 207.23
CAS No.: 29218-27-7
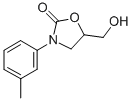
Chemical Name: moclobemide
Molecular Formula: C13H17ClN2O2
Formula Weight: 268.74
CAS No.: 71320-77-9
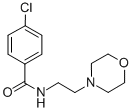
Chemical Name: befloxatone
Molecular Formula: C15H18F3NO5
Formula Weight: 349.303
CAS No.: 134564-82-2
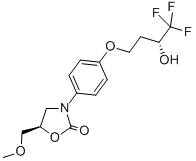
Chemical Name: cimoxatone
Molecular Formula: C19H18N2O4
CAS No.: 73815-11-9
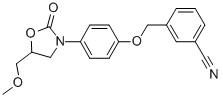
Chemical Name: esuprone
Molecular Formula: C13H14O5S
Formula Weight: 282.315
CAS No.: 91406-11-0
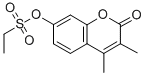
Chemical Name: methylene blue
Molecular Formula: C16H18ClN3S
Formula Weight: 319.85
CAS No.: 61-73-4
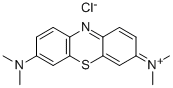
Chemical Name: sercloremine
Molecular Formula: C14H16ClNO
Formula Weight: 249.739
CAS No.: 54403-19-9
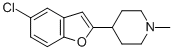
Chemical Name: tetrindole
Molecular Formula: C21H30N2O3S
Formula Weight: 390.54
CAS No.: 135991-95-6
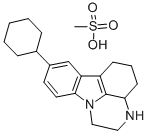
Chemical Name: phenelzine
Molecular Formula: C8H12N2
Formula Weight: 136.19
CAS No.: 51-71-8
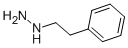
Chemical Name: tranylcypromine
Molecular Formula: C9H11N
Formula Weight: 133.19
CAS No.: 95-62-5

For comparison purposes I'm listing other MAOIs below, including pictures of their structures, if it's useful. Maybe someone can say something about the comparison between their structures and harmine's structure.

*Harmine is the predominant MAOI in Banisteropsis caapi vine.
Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson's disease. Samoylenko, V.; Rahman, M.; Tekwani, B. L.; Tripathi, L. M.; Wang, Y.; Khan, S. I.; Khan, I. A.; Miller, L. S.; Joshi, V. C.; Muhammad, I. Journal of ethnopharmacology. 10/2009; 127(2):357-67. DOI: 10.1016/j.jep.2009.10.030 DOWNLOAD
**Generic names: phenelzine and tranylcypromine.
Chemical Name: brofaremine
Molecular Formula: C14H16BrNO2
C AS No.: 63638-91-5

Chemical Name: caroxazone
Molecular Formula: C10H10N2O3
CAS No.: 18464-39-6

Chemical Name: eprobemide
Molecular Formula: C14H19ClN2O2
Formula Weight: 282.769
CAS No.: 87940-60-1

Chermical Name: amiflamina
Formula Weight: C12H20N2
CAS No.: 77697-37-1

Chemical Name: metralindole
Molecular Formula: C15H17N3O
CAS No.: 54188-38-4

Chemical Name: minaprine
Molecular Formula: C17H24Cl2N4O
Formula Weight: 371.3
CAS No.: 25905-77-5

Chemical Name: pirlindole
Molecular Formula: C16H22N2O3S
Formula Weight: 322.42
CAS No.: 60762-57-4

Chemical Name: toloxatone
Molecular Formula: C11H13NO3
Formula Weight: 207.23
CAS No.: 29218-27-7

Chemical Name: moclobemide
Molecular Formula: C13H17ClN2O2
Formula Weight: 268.74
CAS No.: 71320-77-9

Chemical Name: befloxatone
Molecular Formula: C15H18F3NO5
Formula Weight: 349.303
CAS No.: 134564-82-2

Chemical Name: cimoxatone
Molecular Formula: C19H18N2O4
CAS No.: 73815-11-9

Chemical Name: esuprone
Molecular Formula: C13H14O5S
Formula Weight: 282.315
CAS No.: 91406-11-0

Chemical Name: methylene blue
Molecular Formula: C16H18ClN3S
Formula Weight: 319.85
CAS No.: 61-73-4

Chemical Name: sercloremine
Molecular Formula: C14H16ClNO
Formula Weight: 249.739
CAS No.: 54403-19-9

Chemical Name: tetrindole
Molecular Formula: C21H30N2O3S
Formula Weight: 390.54
CAS No.: 135991-95-6

Chemical Name: phenelzine
Molecular Formula: C8H12N2
Formula Weight: 136.19
CAS No.: 51-71-8
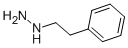
Chemical Name: tranylcypromine
Molecular Formula: C9H11N
Formula Weight: 133.19
CAS No.: 95-62-5

Last edited:





