You're probably right about the particles actually, I guess the wicks are fairly loose and porous since the liquid's pretty thick. I knew about the polarity, it gets a bit complicated to imagine how that might all play out with glycerol and PG though! How do you know PG is less polar?
Hehe, yeah it's hard to think of much that meets those requirements! I think alcohol will work fine, it probably won't need much and I don't think the boiling point difference will matter.
I think I had it wrong about the particles, I was assuming that the insolubles mix with PG to form a suspension, but I later found out the particles eventually settle at the bottom so its not a suspension. I shoulda expected that, in my experience, if somethings completely insoluble in a solvent, it'll remain clumped together, whereas a more soluble material gets dispersed as fine particles. I can't remember the terminology for the different kinds of suspensions, the really fine ones are called colloids, but can't remember the names for the other ones like milk.
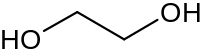
PG is an easy one to think about, just compare ethylene glycol to ethanol. One end of ethanol has that electron rich oxygen atom, while the other end of the molecule is relatively electron deficient so this creates a dipole along the x-axis. Ethylene glycol on the other hand:
doesn't have an electron deficient tail. Both ends are electron rich, so no dipole. Now take glycerol:
no dipole along the x-axis, but that additional OH group on the top of the molecule means theres a dipole along the y-axis. If glycerol had longer carbon chains, then it would be more flexible and could adopt a less polar conformation (staggered conformations aren't always the most stable), but it doesn't so that dipole along the y-axis is there for good. In chemistry theres rarely just one factor involved in a phenomenon, this is no different. From what I remember, ethylene glycol is actually more polar than ethanol. I don't know the reason, but its probably due to greater ability to form hydrogen bonds.
Sorry, I meant to use PG and n-propanol as an example, but the explanation applies equally. PG should be even better at forming hydrogen bonds because long chain length means greater flexibility.
So is the wick the word for that paper stuff inside the cartridge, or is it the tube down the center of it? I looked up e-cig wicks and I heard talk about making your own with silica. I'd be careful there, polars like methanol dissolve silica.