paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
I spent the last few days trying get hold of this paper. Cant even find a doi. seems the journal start indexing 1987 and up (the paper is 1980). I would be most grateful if anybody has access to some sort of electronic version:
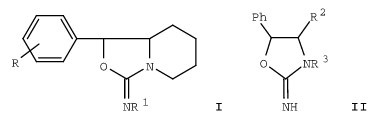
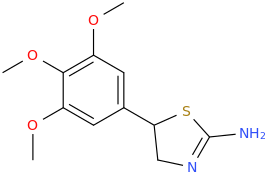
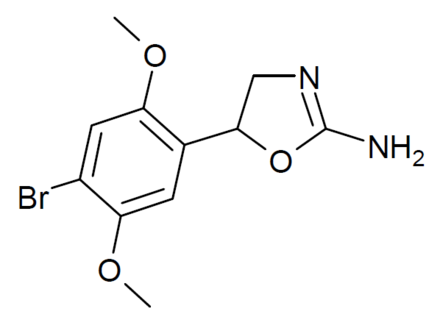
It is SAR of Aminorex (structure II R3R2 = H), 4-MAR (structure II R3 = H and R2 = Me) and cyclic homologs I in vivo! not in vitro monoamines activity data. Both cis and trans as well as their (+) and (-) enantiomers. Really very very interesting! Thanks a lot for your help.
European Journal of Medicinal Chemistry 1980 Vol 15 Issue 2 Pages 111-117.
2. Stereochemistry studies on drugs. 8. 1-Phenyl-3-imino-perhydro-3H-oxazolo(3,4-a)pyridine with blood pressure increasing activity
By Wollweber, H; Hiltmann, R.; Stoepel, K.; Kronenberg. H. G.
Isomers of the iminoperhydrooxazolopyridines I (R = H, 3-Me, 4-Me, 3-OMe, 4-OMe, 3-Cl, 4-Cl; R1 = H, Me) and of the iminooxazolidines II (R2, R3 = H, Me) were prepd. stereospecifically according to literature methods. The antihypotensive and central system stimulant activity of I and II is stereochem.-dependent, (-)-trans-I (R = R1 = H) being most active.

It is SAR of Aminorex (structure II R3R2 = H), 4-MAR (structure II R3 = H and R2 = Me) and cyclic homologs I in vivo! not in vitro monoamines activity data. Both cis and trans as well as their (+) and (-) enantiomers. Really very very interesting! Thanks a lot for your help.