What exactly makes a compound selective dopamine reuptake/releaser or selective serotonin-dopamine reuptake/releaser
devoid of any norepinephrine activity? It is extremelly difficult to separate DAT and NET activity as the two transporters have virtually identical susbsttrate requirements. More difficult still is separating NET from DAT activities while retaning SERT activity intact. They are only 2 known compounds that are
selective SDRI.
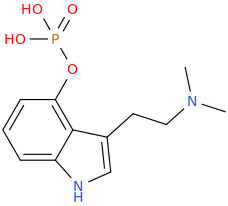
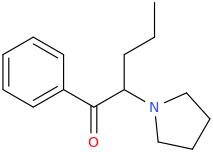
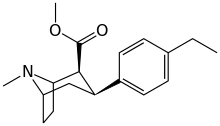
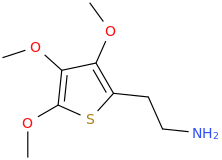
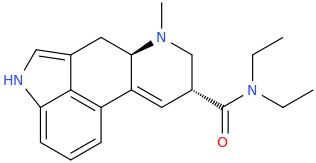
RTI-83
Ki values DAT(15nM) SERT(7.1nM) and NET(28,000nM).
and
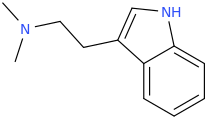
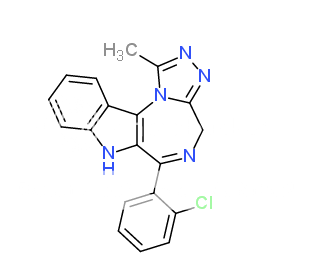
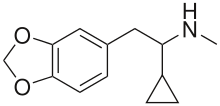
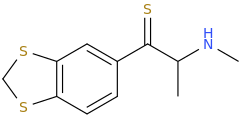
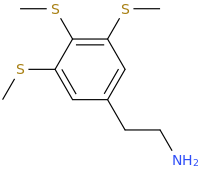
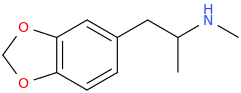
UWA-101
My question is: those 2 molecules look so dissimilar it is hard to see what make them selective for DAT and SERT as opposed to NET. Is there a common motif I can't put my finger on that kills binding at NET transporter? second question: afaik there is no erowid experience reports on selective SDRI those, especailly the MDMA analog UWA-101 that is apparently now availaible in NZ/australia? correct me if I am wrong.
Comparison of SDRIs to SNDRIs
https://en.wikipedia.org/wiki/Serotonin-dopamine_reuptake_inhibitor




![(−)-(6aR,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol.png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%28%E2%88%92%29-%286aR%2C10aR%29-6%2C6%2C9-Trimethyl-3-pentyl-6a%2C7%2C8%2C10a-tetrahydro-6H-benzo%5Bc%5Dchromen-1-ol.png&hash=0477716b648c8e4a2ffa0733baa704ed)