sekio
Bluelight Crew
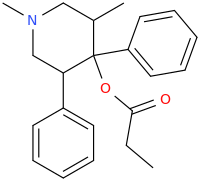
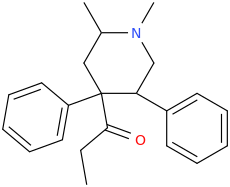
Cyclic analogs of propoxyphene / methadone as substituted pethidine / ketobemidone / prodine analogs
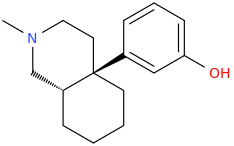
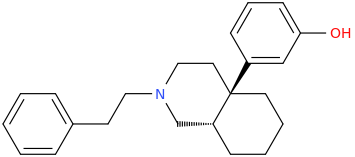
US4219652A compounds, opioid analgesics
![1,2,3,4,5,6,7-heptahydro-N-methyl-9-hydroxy-Benzo[b]isoquinolino[4a,4-d]furan.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1%2C2%2C3%2C4%2C5%2C6%2C7-heptahydro-N-methyl-9-hydroxy-Benzo%5Bb%5Disoquinolino%5B4a%2C4-d%5Dfuran.png&hash=d8184af3fdc0b3992a83dd1e05eca343)
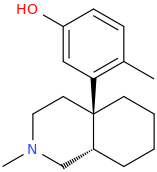
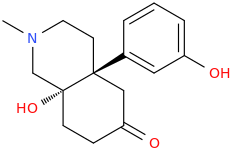
Related
![1,2,3,4,5,6,7-heptahydro-N-methyl-2-oxo-4a,9-dihydroxy-12-methyl-Benzo[b]isoquinolino[4a,4-d]furan.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1%2C2%2C3%2C4%2C5%2C6%2C7-heptahydro-N-methyl-2-oxo-4a%2C9-dihydroxy-12-methyl-Benzo%5Bb%5Disoquinolino%5B4a%2C4-d%5Dfuran.png&hash=8a2156da7dc7e77e6d0262444412254a)
9,10-seco-oxymorphone
Last edited:
N&PD Moderators: Skorpio | someguyontheinternet




![1,2,3,4,5,6,7-heptahydro-N-methyl-9-hydroxy-Benzo[b]isoquinolino[4a,4-d]furan.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1%2C2%2C3%2C4%2C5%2C6%2C7-heptahydro-N-methyl-9-hydroxy-Benzo%5Bb%5Disoquinolino%5B4a%2C4-d%5Dfuran.png&hash=d8184af3fdc0b3992a83dd1e05eca343)


![1,2,3,4,5,6,7-heptahydro-N-methyl-2-oxo-4a,9-dihydroxy-12-methyl-Benzo[b]isoquinolino[4a,4-d]furan.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1%2C2%2C3%2C4%2C5%2C6%2C7-heptahydro-N-methyl-2-oxo-4a%2C9-dihydroxy-12-methyl-Benzo%5Bb%5Disoquinolino%5B4a%2C4-d%5Dfuran.png&hash=8a2156da7dc7e77e6d0262444412254a)
^ Wouldn't that (the compound above) be hydrolyzed immediately like most acid anhydrides?
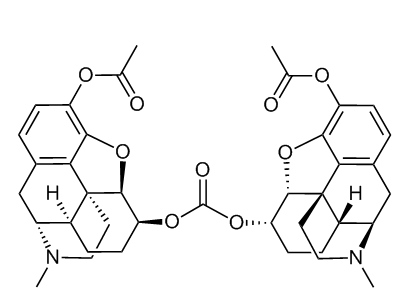

from US20050154002A1, carbonate ester prodrug of 3-Ac-dihydromorphine
This brings up an interesting legal question: is the dimer of a compound an analogue of it? That would be an interesting case to see!
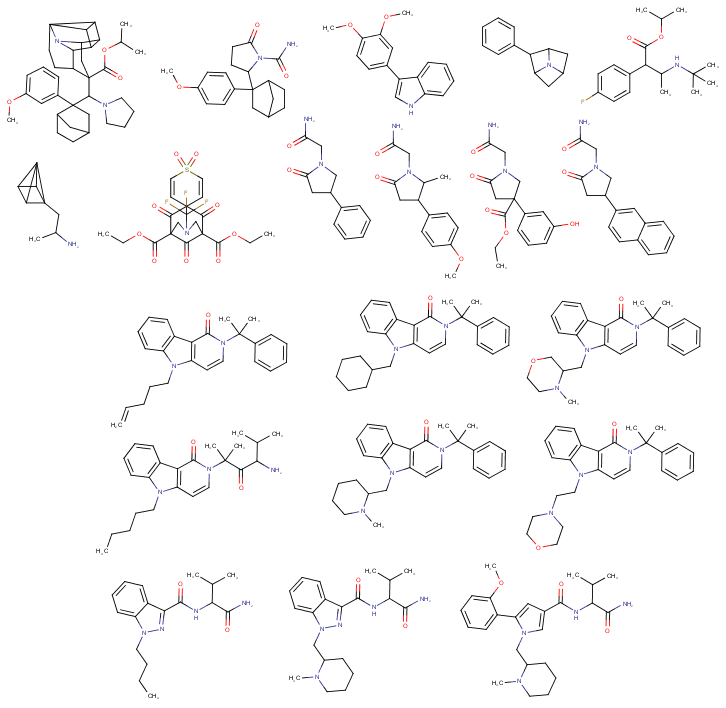
simply put, the pentenyl chain 'looks' a lot like the pentyl chain, and is still nonreactive enough that it doesn't mess with the drug's binding or half life by too much.Can anyone explain where the difference between a pentyl-chain like usually found in cannabinoids and a pentene-yl chain like you can find in some of today's overly potent cannabinoids
why aren't they more widely used? Seems like a simple change in chemistry design. (For example there is a pentene-yl chain on the third cannbinoid above the bottom left one)
For example, exposure to acids like HCl can result in hydrohalogenations (turning the alkene into a saturated haloalkane)