The stats! :* Links to the results last for around a month, not more, as I noticed with the posts of my "Gaffy's analog act". Replacing them on the usual would be a real hassle. What i can do is post just the image and comment on the % of binding probability underneath!
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
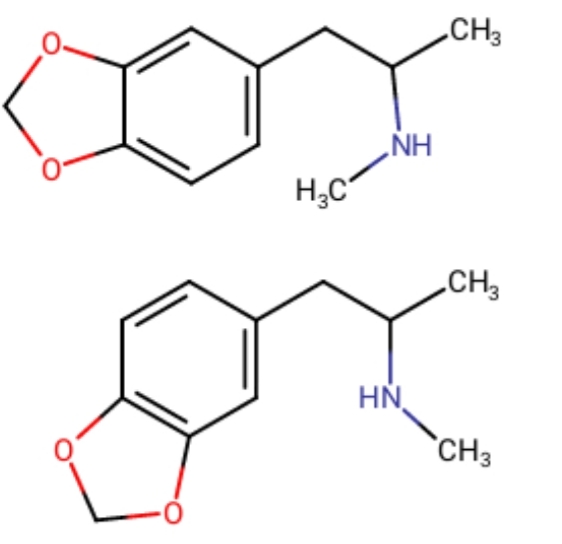
So I was wondering, just how much difference is there between 3,4 MD and 4,5 MD. As you can see in the picture, the double carbon bond position differes from one to another's, maybe that's what's wrong with today's MDMA?
Last edited by a moderator:
Bagseed
Bluelighter
there is no real carbon double bond because in an aromatic ring system, all the electrons are delocalized....
Nagelfar
Bluelight Crew
Yes, piperidine homologues of cocaine have been studied multiple times.^ I could swear a very similar compound was in Lenz's Opiates. Don't think they were more effective than pethidine though. I know that the meta-isomer of pethidine is less effective.
Thanks bagseed, what is your explanation for the difference between MDMA made from safrole and the one made from MDP2P? One is euphoric, stimulant and enjoyable, the other is stressful, disphoric and makes you tired.there is no real carbon double bond because in an aromatic ring system, all the electrons are delocalized....
There's already a thread for this kind of speculation, please take your discussion there.Thanks bagseed, what is your explanation for the difference between MDMA made from safrole and the one made from MDP2P? One is euphoric, stimulant and enjoyable, the other is stressful, disphoric and makes you tired.
sekio
Bluelight Crew
3,4 MDMA and 4,5 MDMA are actually the same molecule. Playing with molecular models should establish that.
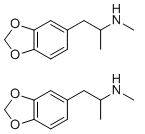
These two MDMA molecules were each made by a different synthesis, can you tell which?

These two MDMA molecules were each made by a different synthesis, can you tell which?
Does the angular curve inbetween the carbons then explain the difference between MDMA made from MDP2P and MDMA made from safrole? Just like Meth made from Ephedrine being like 10x times better than meth made from p2p.
Any idea for another M1-4 compound? Different structure?
Any idea for another M1-4 compound? Different structure?
sekio
Bluelight Crew
Does the angular curve inbetween the carbons then explain the difference between MDMA made from MDP2P and MDMA made from safrole?
No. You seem to forget that most MDP2P is made from safrole. In any case, racemic MDMA is racemic MDMA. It would do you good to learn about the 3d structures of organic chemicals: the little line drawings are not 1:1 representations of how these compounds exist in space.
Ephedrine is a chirally pure natural product, so meth produced from it is 95% dextro-methamphetamine. Methamphetamine from P2P is only 50% as it's racemic.Just like Meth made from Ephedrine being like 10x times better than meth made from p2p.
Any idea for another M1-4 compound?
Why, do you like salivation? The effects of muscarinics are not known to be very pleasant.
I suspect your compound is actually an antimuscarinic, i.e. atropine-like. Those are not known to be pleasant. Compare the structure of BZ and others. The dudes at Edgewood Arsenal pretty much beat you to the punch for antimuscarinics.
I suspect your compound is actually an antimuscarinic, i.e. atropine-like. Those are not known to be pleasant. Compare the structure of BZ and others. The dudes at Edgewood Arsenal pretty much beat you to the punch for antimuscarinics.
Do you have an example?
sekio
Bluelight Crew
I am suprised you aren't familiar with atropine-like deleriants? The muscarinic antagonism is part of what produces the CNS deleriant properties.
Some antimuscarinics are are useful agents to stop things like excess salivation but must be carefully dosed to avoid strange hallucinogenic effects.
BZ was developed as an incapacitating agent for that reason; a dose is measured in the low milligrams and will lead to a 2-3 day long delerious fugue state.
Similar effects are noted in intoxication with solanaceous plants like henbane, datura, brugmansia, etc. and also from drugs with anticholinergic acitivity like diphenhydramine.

Funny enough, one of the "antidotes" to BZ toxicity is sarin or similar nerve gas in a very carefully measured dose... the idea being that the nerve gas will inactivate some of the acetylcholinesterase enzymes, increasing cholinergic signalling, and overcoming the blockade of muscarinic receptors by BZ. Obviously this is not clinically reccomended but...
Some antimuscarinics are are useful agents to stop things like excess salivation but must be carefully dosed to avoid strange hallucinogenic effects.
BZ was developed as an incapacitating agent for that reason; a dose is measured in the low milligrams and will lead to a 2-3 day long delerious fugue state.
Similar effects are noted in intoxication with solanaceous plants like henbane, datura, brugmansia, etc. and also from drugs with anticholinergic acitivity like diphenhydramine.

Funny enough, one of the "antidotes" to BZ toxicity is sarin or similar nerve gas in a very carefully measured dose... the idea being that the nerve gas will inactivate some of the acetylcholinesterase enzymes, increasing cholinergic signalling, and overcoming the blockade of muscarinic receptors by BZ. Obviously this is not clinically reccomended but...
Found a cool javascript chemdraw thing... used to have to pirate the software to get features like structure to name. Anyway smoked some a-pcyp and started thinking about modifications to it... its a very potent stimulant. One of the most potent ones i've tried, almost like MDPV..
sekio
Bluelight Crew
OK, as of today, no nore STP posts here, please. Take those to the molecular doodles thread.
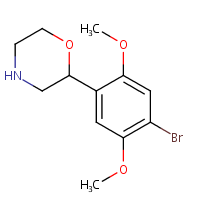
Apparently this is active at 5HT2a?

Apparently this is active at 5HT2a?
Last edited:
Is just using STP to use ChemAxon ok? Like in my post above?
As for unlikely Psychedelics, here's one I drew some time ago:
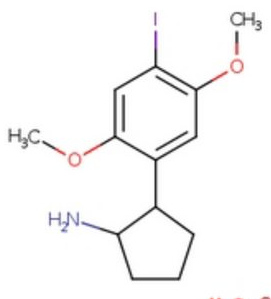
Btw Sekio, is the IUPAC in my signature correct? If not can you name one please?
As for unlikely Psychedelics, here's one I drew some time ago:

Btw Sekio, is the IUPAC in my signature correct? If not can you name one please?
Last edited by a moderator:
sekio
Bluelight Crew
Chemdraw chokes on it, and it doesn't look like a valid IUPAC name, no.
Is this what you meant?
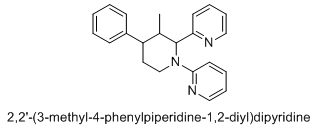
The 3 chiral centers are 2, 3, & 4. Usually if you have a racemic compound specifying (RS) is unneccesary - any unspecified chiral center is assumed to be racemic.
Also, I would prefer you stick to smaller images posted using the [img][/img] tag.
Is this what you meant?

The 3 chiral centers are 2, 3, & 4. Usually if you have a racemic compound specifying (RS) is unneccesary - any unspecified chiral center is assumed to be racemic.
Also, I would prefer you stick to smaller images posted using the [img][/img] tag.
I meant the picture I committed to naming GAFFY in the Name-A-Molecule thread ^^ This one:
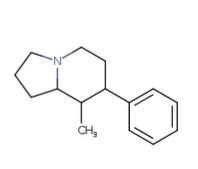
A-AVP
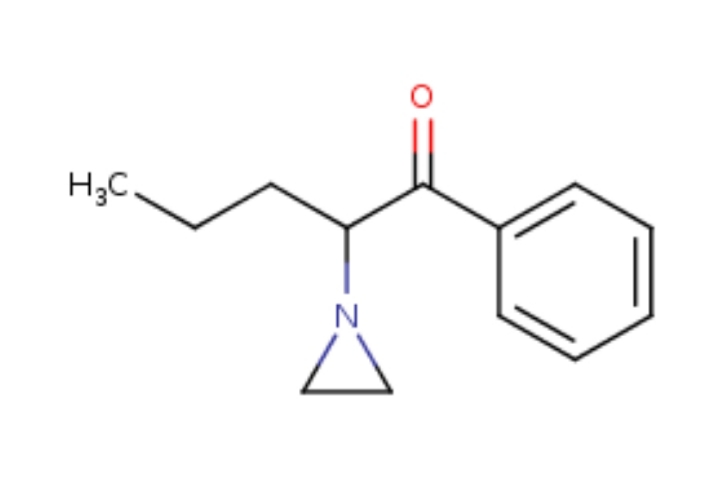
Alpha-AziridineValeroPhenone
Edit:I just realised the difference between pyrrolydine and pyridine.. I will update that asap!
Done

A-AVP

Alpha-AziridineValeroPhenone
Edit:I just realised the difference between pyrrolydine and pyridine.. I will update that asap!
Done
Last edited:
- Status
- Not open for further replies.





