Could yhat technetium cocaine be responsible for hallucinations? Or kinesic capabilitys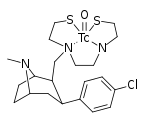
Technetium? In my cocaine? It's more likely than you think.
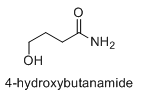
Also, does anyone know about the pharmacology of GHB-amide? Presumably, it would be metabolically converted to GHB, but does it also have activity on its own?
I know the cyclic lactam is not anywhere near GBL in activity, as I have never hard of anyone abusing pyrrolidinone.
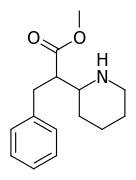
And Gaffy - this compound below is known in the literature, and looks similar to your doodles, but unfortunately has a >5000 nM DAT Ki. In other words, maybe blazeocaine is not so hot after all.
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
sekio
Bluelight Crew
What makes you think it would do that? As far as I can tell, it's just another cocaine analogue, albeit one with a tethered radionuclide on it. Probably just another variety of stimulant.
You could probably use it to take some cool pictures that show you where your dopamine transporters are located though.
You could probably use it to take some cool pictures that show you where your dopamine transporters are located though.
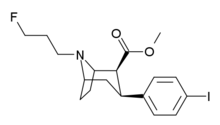
Bulk cocaine must be 1 to 1, maybe less, for 4-fluorococaine to be just 3 to one. This isn't a price naming, just some random stats
As for nuclear receptor activity, I sometimes feel like a prophet making designs of molecules always finding the right one fitting the situation:
 swisstargetprediction.ch
swisstargetprediction.ch
Triple nuclear receptors
As for nuclear receptor activity, I sometimes feel like a prophet making designs of molecules always finding the right one fitting the situation:
SwissTargetPrediction
Triple nuclear receptors
Last edited:
sekio
Bluelight Crew
Because it's emitting gamma
Why do you think gamma radiation would give you hallucinations or otherwise? Many gamma-emitting radiopharmaceuticals are used for various different imaging uses, and to the best of my knowledge, the radiation doses are too low to cause any major damage or strange effects.
A related radiopharmaceutical, ioflupane, brand name DATScan, is used for a similar purpose, imaging the DAT protiens. It uses radioactive iodine instead of technetium.
I think the dosage is below that needed for a reliable stimulant effect, but it still produces some mild mental changes, as described below.
Common side effects of ioflupane (123I) are headache, vertigo, increased appetite and formication (crawling sensation on the skin). Less than 1% of patients experience pain at the injection site.
The radiation risks are reported as low. The committed effective dose for a single investigation on a 70 kg individual is 4.6 mSv. Pregnant patients should not undergo the test. It is not known if 123I-ioflupane is secreted in breast milk however it is recommended that breastfeeding is interrupted for three days after administration.
Bulk cocaine must be 1 to 1, maybe less, for 4-fluorococaine to be just 3 to one. This isn't a price naming, just some random stats
Stats about what? If you're making some obtuse reference to pricing, then on paper 4-fluorococaine should not be that much more expensive in bulk, if you can come up with a nice way to cleave the benzoyl ester selectively then you can easily make it from cocaine, or from ecgonine.
Nagelfar
Bluelight Crew
Gaffy, as sekio said, it needs a referrece to be included in WP, otherwise it violates WP's "original research" rules. (people dig into WP as inaccurate, but what you need to pay attention to are the links to the references and trust those)
Anyway, I am limited to my phone right now, and I cannot so much as cut and paste a link with it to add an image here.
Anyway, I am limited to my phone right now, and I cannot so much as cut and paste a link with it to add an image here.
sekio
Bluelight Crew
As for nuclear receptor activity, I sometimes feel like a prophet making designs of molecules always finding the right one fitting the situation:
Another interpretation might be that any old crap you punch into STP has a reasonably good chance of having a high probability at at least one receptor, especailly small "druglike" compounds that adhere to Rule of 5.
I don't get where you infer the activity of that compound at all. The probability rankings are all very low. If anything, the 2nd and 3rd rank are COX1 and COX2, meaning it's an anti-inflammatory. But those have equally as bad probability.
Nagelfar
Bluelight Crew
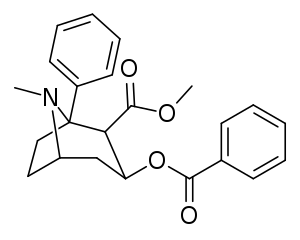
Found out how to paste with my phone (it's not easy, don't expect a lot from me)
The above is a phenyl C1 substitution of cocaine, which not only has greater affinity than cocaine at DAT, but is also a more potent sodium channel blocker (topical/local anesthetic). I wonder what a phenyltropane version would turn out as.
Last edited by a moderator:
Nagelfar
Bluelight Crew
^not showing up? It showed up in my text box! Oh well.
sekio
Bluelight Crew
Like a ghost in the mid-morning, I swooped in and fixed it for you. The forum software doesn't like SVG files, it needs to be a bitmap format - so use the links at the bottom of a Wiki image.
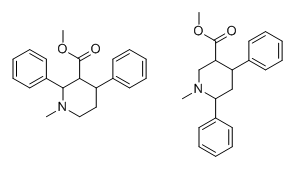
Reminds me of the very simple diphenyltropanes.

I wonder what a phenyltropane version would turn out as.
Reminds me of the very simple diphenyltropanes.
Nagelfar
Bluelight Crew
Whoops, think I edited it in the meantime. But thank you sekioLike a ghost in the mid-morning, I swooped in and fixed it for you. The forum software doesn't like SVG files, it needs to be a bitmap format - so use the links at the bottom of a Wiki image.

It's Funny how a 3-OH on that dimethylpentanephenylpiperidine changes stats to opioid action
Please excuse me, I've had 19 alcohol doses today. Confinement isn't really a good thing to me, I normally get high..
Nagelfar, take a look at these: I prefer thse to cocaine as they are faster to draw but still reflect the overall pharmacology (do they?)
As in your picture, one carbon away from the carbomethoxy- and from the N-methyl
On the other side, one carbon away from the n-Methyl
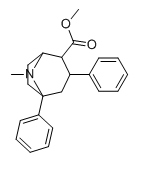
Differences in SER binding probability, approx same dopa. Have you got any references on the second substitution done on cocaine ?
Here done with phenyltropane
And to take our mind off things,
OH-JWH018
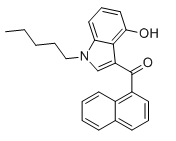
Bulk cocaine must be 1 to 1, maybe less, for 4-fluorococaine to be just 3 to one. This isn't a price naming, just some random stats
As for nuclear receptor activity, I sometimes feel like a prophet making designs of molecules always finding the right one fitting the situation:
SwissTargetPrediction
swisstargetprediction.ch
Triple nuclear receptors
Please excuse me, I've had 19 alcohol doses today. Confinement isn't really a good thing to me, I normally get high..
Nagelfar, take a look at these: I prefer thse to cocaine as they are faster to draw but still reflect the overall pharmacology (do they?)
As in your picture, one carbon away from the carbomethoxy- and from the N-methyl
On the other side, one carbon away from the n-Methyl

Differences in SER binding probability, approx same dopa. Have you got any references on the second substitution done on cocaine ?
Here done with phenyltropane

And to take our mind off things,
OH-JWH018

Last edited by a moderator:
Nagelfar
Bluelight Crew
Except for your one, they don't have the bridge that makes the boat formation in 3D. One that does puts phenyl on at the five position, quite different from the one position. From studies every change at the six or seven position hinders binding to some degree, since it's opposite the carbmethoxy side I'd figure it would hinder binding also.
sekio
Bluelight Crew
moved to the doodles thread
sekio
Bluelight Crew
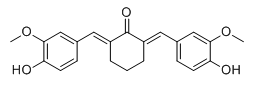
Cyclovalone, which is "a choleretic and cholagogic agent", or so I'm told. An analog of the active principle of tumeric - curcumin.
Exercise fot 1st year organic synthesis students: devise a 1 step synthesis from commercial reagents.
I cheated, but maybe vanilline and cyclohexylone with HCl and dimethylamine? I absolutely do not understand how though.
STP doesn't seem to differ between agonism and antagonism, as I think we can all agree this should act as a antipsychotic, thus an antagonist, but it isn't clarified, which I just noticed!^^
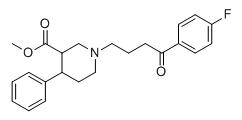
1-Ethyl-3-CarboEthoxy-4-Phenyl-Piperidine. Very high mu binding affinity (around 80%, highest I've seen), higher than ketobemifone, the holy grail of opioids; higher than for dopa, as well as a higher binding probability for M1-4 (×2 at least) than its father compound 1-Methyl-3-CarboMethoxy-4-Phenyl-Piperidine wich has a 100% binding probability for dopa but only around 20 for mu.
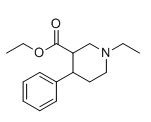
STP doesn't seem to differ between agonism and antagonism, as I think we can all agree this should act as a antipsychotic, thus an antagonist, but it isn't clarified, which I just noticed!^^

1-Ethyl-3-CarboEthoxy-4-Phenyl-Piperidine. Very high mu binding affinity (around 80%, highest I've seen), higher than ketobemifone, the holy grail of opioids; higher than for dopa, as well as a higher binding probability for M1-4 (×2 at least) than its father compound 1-Methyl-3-CarboMethoxy-4-Phenyl-Piperidine wich has a 100% binding probability for dopa but only around 20 for mu.

Last edited by a moderator:
sekio
Bluelight Crew
^ I could swear a very similar compound was in Lenz's Opiates. Don't think they were more effective than pethidine though. I know that the meta-isomer of pethidine is less effective.
sekio
Bluelight Crew
I am going to go through this thread and replace the bulky STP images with links to the STP results and a much smaller image that just shows the stucture.
In future, can we please not post the whole STP results in an image? I am almost considering restricting all STP discussion to a single thread, because it dorwns out the more academic discussion of everything else...
In future, can we please not post the whole STP results in an image? I am almost considering restricting all STP discussion to a single thread, because it dorwns out the more academic discussion of everything else...
- Status
- Not open for further replies.




