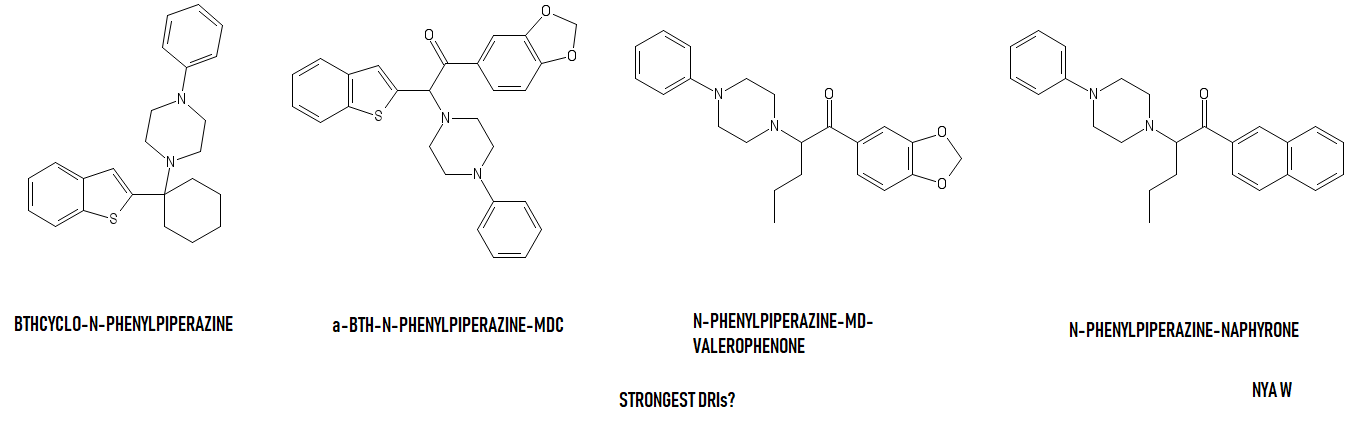
Sorry about that. I only have a very basic (if not plain bad) chemistry level.
No reason to apologize.

I was genuinely curious as to why you would assume that three bonds on the sulfur atom would make it more stable, so I could better explain it to you.
Maybe this will make it easier to understand:
As I've said, sulfur has 6 electrons in its outer ("valence") shell. Usually, atoms in compounds will enter a certain number of bonds with other atoms in order to achieve a shell with 8 electrons in it.
Take, for example, hydrogen sulfide (H2S): The sulfur enters two of its electrons into single bonds with hydrogens, so it now has 2 x 2 electrons in the S-H bonds, in addition to 2 pairs of electrons not involved in bonds (so-called "lone pairs").
Since (2x2) + (2x2) = 8, it has now achieved the desired 8-electron ("octet") configuration.
Compare this to, for example, carbon or nitrogen:
Carbon has 4 electrons, so it going to try and enter bonds to gain 4 more electrons; nitrogen has 5, so nitrogen needs to form 3 bonds to complete its octet:
Thus the formula for methane is CH4, and that of ammonia is NH3.
In benzene, each carbon is connected to one carbon atom via a single bond, and to another via a double bond. Its fourth bond is to hydrogen, but carbon-hydrogen-bonds are typically not drawn in a skeletal formula so as not to make it overly complicated.
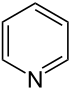
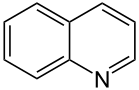
By replacing one of the carbon atoms in benzene with nitrogen, we get pyridine. Note that here, you'd have to draw a hydrogen atom if there was one connected to the nitrogen - but there isn't, because the nitrogen is quite happy with the 3 bonds (a single bond and a double bond) it has formed with the adjacent carbons, giving it the desired 8-electron-configuration.
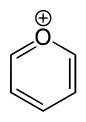
Now, it is possible to have a compound where the sulfur is connected to the adjacent carbon atoms via 3 bonds, namely by removing one of its electrons. With 5 electrons in its valence shell, it is now "isoelectronic" with nitrogen, and will thus bond to the adjacent carbons just like the nitrogen in pyridine. Removing the electron leaves it with a positive charge; however, it can be stabilized by forming a salt with a negatively charged counterion (e.g. chloride). The image below shows a pyrylium cation; replace the oxygen with sulfur, and you'd have the thiopyrylium cation.