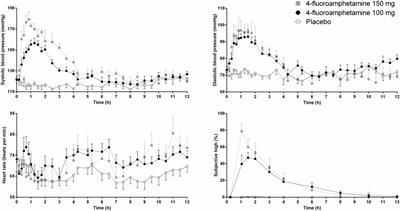
hydroazuanacaine
bluelighter
- Joined
- May 17, 2007
- Messages
- 8,497
if you search, google will turn up a lot of results about fluorinated amphetamines being more neurotoxic and/or more cardiotoxic than plain amphetamine. these results are largely reddit posts and the closest thing to a study being cited is talking about a 4-fa study about serotonin neurotoxicity that's never actually linked to and that people argue about the results of.
i recently discovered 2-fa and 2-fma. they are like amphetamine light, and i like them very much. especially 2-fa, which is so cool and gentle it's hard to believe it's an amphetamine. 2-fma is a little bit more like classic dextroamphetamine in that it has some more umph to it, but still not on the same level adderall. so 2-fma is good for when you need the drug to last a few hours while you get something done; 2-fa when your project is only an hour or two. they feel even less toxic than adderall, but i know that doesn't mean anything.
is there any legitimacy to these online repetitions of fluorinated amphetamines being more toxic than amphetamine in general? i realize there is no definite answer because 2-fa and 2-fma have not been studied like amphetamine. i'm hoping people like @sekio could weigh in and let me know if there is a foundation to these rumors that i should look into? or if people are simply repeating what they heard and it's not supported by anything meaningful?
thank you!
i recently discovered 2-fa and 2-fma. they are like amphetamine light, and i like them very much. especially 2-fa, which is so cool and gentle it's hard to believe it's an amphetamine. 2-fma is a little bit more like classic dextroamphetamine in that it has some more umph to it, but still not on the same level adderall. so 2-fma is good for when you need the drug to last a few hours while you get something done; 2-fa when your project is only an hour or two. they feel even less toxic than adderall, but i know that doesn't mean anything.
is there any legitimacy to these online repetitions of fluorinated amphetamines being more toxic than amphetamine in general? i realize there is no definite answer because 2-fa and 2-fma have not been studied like amphetamine. i'm hoping people like @sekio could weigh in and let me know if there is a foundation to these rumors that i should look into? or if people are simply repeating what they heard and it's not supported by anything meaningful?
thank you!
Last edited: