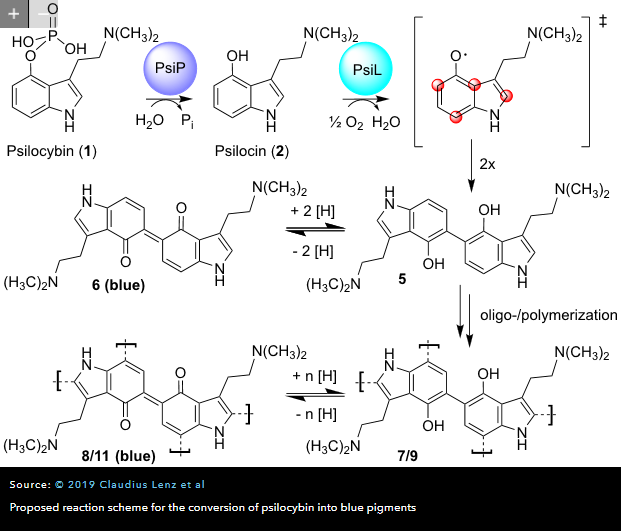
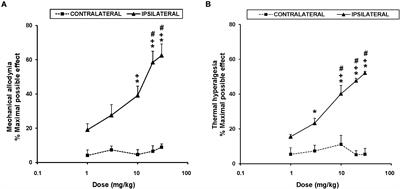
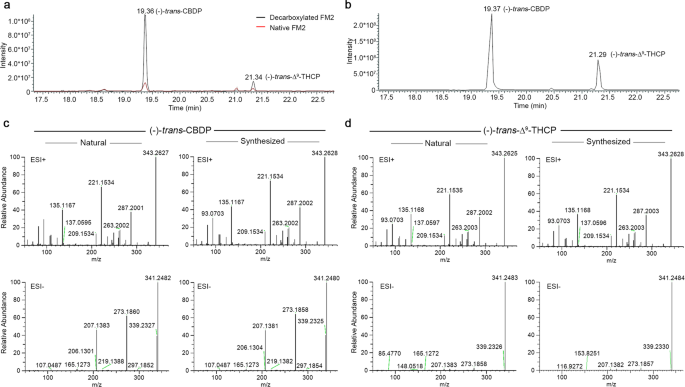
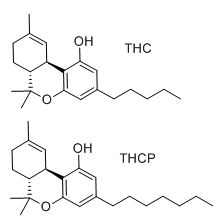
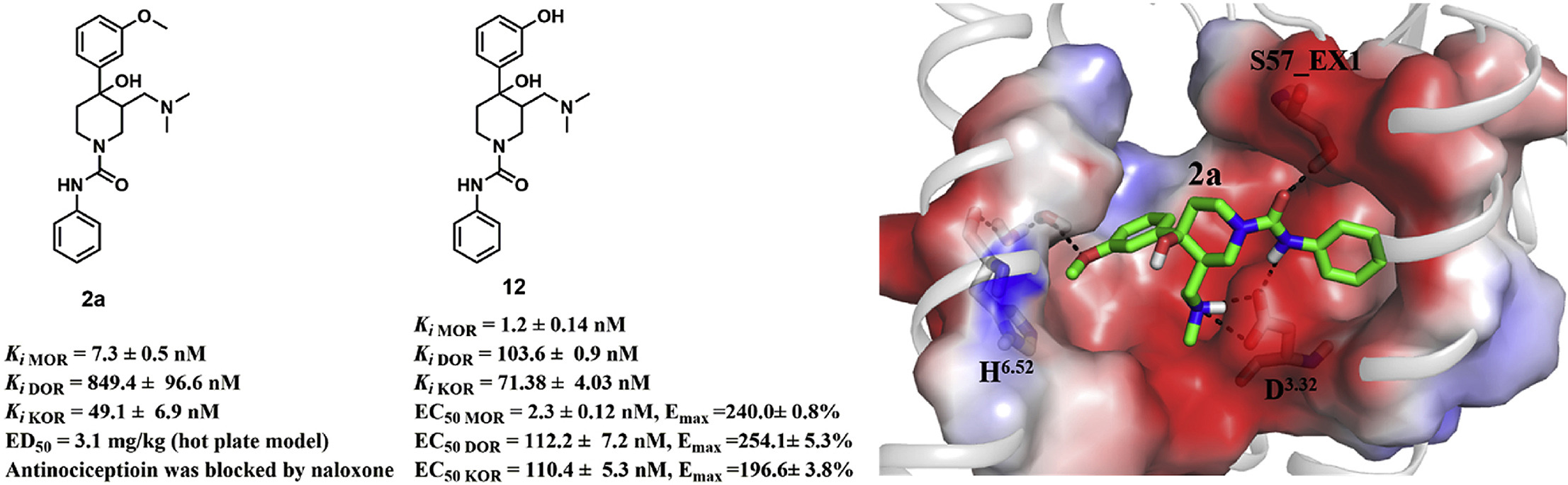
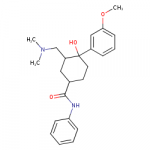
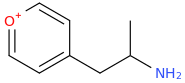
Cortexiphan
Bluelighter
- Joined
- Aug 25, 2011
- Messages
- 99
DARK Classics in Chemical Neuroscience: NBOMes
Authors: Christian B. M. Poulie, Anders A. Jensen, Adam L. Halberstadt, Jesper L. Kristensen*
ACS Chem. Neurosci. 2019,
Publication Date:October 28, 2019
https://doi.org/10.1021/acschemneuro.9b00528

Authors: Christian B. M. Poulie, Anders A. Jensen, Adam L. Halberstadt, Jesper L. Kristensen*
ACS Chem. Neurosci. 2019,
Publication Date:October 28, 2019
https://doi.org/10.1021/acschemneuro.9b00528
| N-Benzylphenethylamines, commonly known as NBOMes, are synthetic psychedelic compounds derived from the phenethylamine class of psychedelics (2C-X compounds), which originally have been derived from the naturally occurring alkaloid mescaline. Analogously to their parent compounds and other classical psychedelics, such as psilocybin and lysergic acid diethylamide (LSD), NBOMes are believed to exert their main pharmacological effects through activation of serotonin 2A (5-HT2A) receptors. Since their introduction as New Psychoactive Substances (NPSs) in 2010, NBOMes have been widely used for recreational purposes; this has resulted in numerous cases of acute toxicity, sometimes with lethal outcomes, leading to the classification of several NBOMes as Schedule I substances in 2013. However, in addition to their recreational use, the NBOMe class has yielded several important biochemical tools, including [11C]Cimbi-36, which is now being used in positron emission tomography (PET) studies of the 5-HT2A and 5-HT2C receptors in the mammalian brain, and 25CN-NBOH, one of the most selective 5-HT2A receptor agonists developed to date. In this Review, the history, chemistry, structure–activity relationships, ADME (absorption, distribution, metabolism, and excretion) properties, and safety profiles of NBOMes will be outlined and discussed. |