paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
does anybody have any info on this compound and related series ?
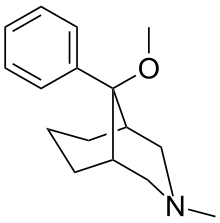
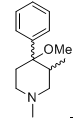
they're a series of meperidine-type analogs bridged at 3-5 by a propane. wiki doesn't say much. from what I get, this compound is about similar analgesic potency as morphine (rats, mice and rhesus). Now, if you add a hydroxy meta on the phenyl ring, the potency jacks up to 500 times morphine. change the stereochemistry at the benzylic carbon (from beta shown->alfa conformer so the phenyl is axial), the potency shoots up whooping 1200x morphine. Replace the N-methyl by phenethyl (beta conformer), the potency goes to the roof about 2500x morphine. I would appreciate your input, in case anybody has come across some old (or not old) literature on those..thx
edit: wiki entry says it was developed in US that's not correct; it was developed in Japan not the US. The harvard study they reference is a confirmation study of the Jpn study. it even does cite the japanese papers.. kind of disingenious to claim is was developed in the US (credit where credit is due I've been taught)

they're a series of meperidine-type analogs bridged at 3-5 by a propane. wiki doesn't say much. from what I get, this compound is about similar analgesic potency as morphine (rats, mice and rhesus). Now, if you add a hydroxy meta on the phenyl ring, the potency jacks up to 500 times morphine. change the stereochemistry at the benzylic carbon (from beta shown->alfa conformer so the phenyl is axial), the potency shoots up whooping 1200x morphine. Replace the N-methyl by phenethyl (beta conformer), the potency goes to the roof about 2500x morphine. I would appreciate your input, in case anybody has come across some old (or not old) literature on those..thx
edit: wiki entry says it was developed in US that's not correct; it was developed in Japan not the US. The harvard study they reference is a confirmation study of the Jpn study. it even does cite the japanese papers.. kind of disingenious to claim is was developed in the US (credit where credit is due I've been taught)
Last edited: